Page 371 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 371
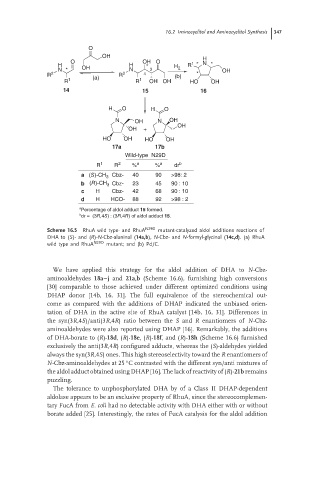
16.2 Iminocyclitol and Aminocyclitol Synthesis 347
O
OH H
O OH O
H H * * H R 1 * * N * *
N * * OH N 3 2 OH
R 2 (a) R 2 4 (b) * *
R 1 R 1 OH OH HO OH
14 15 16
H O H O
N OH N OH
OH
OH +
HO OH HO OH
17a 17b
Wild-type N29D
R 1 R 2 % a % a dr b
Cbz- 40 90 >98: 2
a (S)-CH 3
b (R)-CH 3 Cbz- 23 45 90 : 10
c H Cbz- 42 68 90 : 10
d H HCO- 88 92 >98 : 2
a
Percentage of aldol adduct 15 formed.
b
dr = (3R,4S) : (3R,4R) of aldol adduct 15.
Scheme 16.5 RhuA wild type- and RhuA N29D mutant-catalyzed aldol additions reactions of
DHA to (S)- and (R)-N-Cbz-alaninal (14a,b), N-Cbz- and N-formyl-glycinal (14c,d).(a) RhuA
wild type and RhuA N29D mutant; and (b) Pd/C.
We have applied this strategy for the aldol addition of DHA to N-Cbz-
aminoaldehydes 18a–j and 21a,b (Scheme 16.6), furnishing high conversions
[30] comparable to those achieved under different optimized conditions using
DHAP donor [14b, 16, 31]. The full equivalence of the stereochemical out-
come as compared with the additions of DHAP indicated the unbiased orien-
tation of DHA in the active site of RhuA catalyst [14b, 16, 31]. Differences in
the syn(3R,4S)/anti(3R,4R) ratio between the S and R enantiomers of N-Cbz-
aminoaldehydes were also reported using DHAP [16]. Remarkably, the additions
of DHA-borate to (R)-18d,(R)-18e,(R)-18f,and (R)-18h (Scheme 16.6) furnished
exclusively the anti(3R,4R) configured adducts, whereas the (S)-aldehydes yielded
always the syn(3R,4S) ones. This high stereoselectivity toward the R enantiomers of
◦
N-Cbz-aminoaldehydes at 25 C contrasted with the different syn/anti mixtures of
the aldol adduct obtained using DHAP [16]. The lack of reactivity of (R)-21b remains
puzzling.
The tolerance to unphosphorylated DHA by of a Class II DHAP-dependent
aldolase appears to be an exclusive property of RhuA, since the stereocomplemen-
tary FucA from E. coli had no detectable activity with DHA either with or without
borate added [25]. Interestingly, the rates of FucA catalysis for the aldol addition