Page 375 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 375
16.3 Carbohydrates and Other Polyhydroxylated Compounds 351
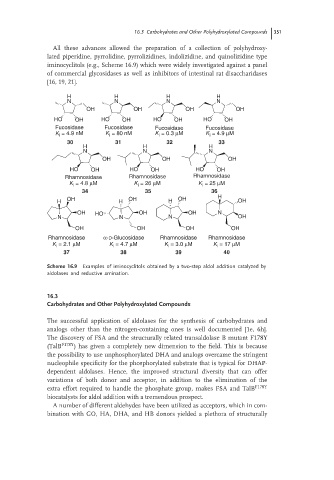
All these advances allowed the preparation of a collection of polyhydroxy-
lated piperidine, pyrrolidine, pyrrolizidines, indolizidine, and quinolizidine type
iminocyclitols (e.g., Scheme 16.9) which were widely investigated against a panel
of commercial glycosidases as well as inhibitors of intestinal rat disaccharidases
[16, 19, 21].
H H H H
N N N N
OH OH OH OH
HO OH HO OH HO OH HO OH
Fucosidase Fucosidase Fucosidase Fucosidase
= 4.9 nM = 80 nM K = 0.3 μM K = 4.9 μM
K i K i i i
30 31 32 33
H H H
N N N
OH OH OH
HO OH HO OH HO OH
Rhamnosidase Rhamnosidase Rhamnosidase
= 4.8 μM K = 26 μM K = 25 μM
K i i i
34 35 36
OH OH OH H
H H H OH
OH HO OH OH N
N N N OH
OH OH OH OH
Rhamnosidase α-D-Glucosidase Rhamnosidase Rhamnosidase
K i = 2.1 μM K i = 4.7 μM K i = 3.0 μM K i = 17 μM
37 38 39 40
Scheme 16.9 Examples of iminocyclitols obtained by a two-step aldol addition catalyzed by
aldolases and reductive amination.
16.3
Carbohydrates and Other Polyhydroxylated Compounds
The successful application of aldolases for the synthesis of carbohydrates and
analogs other than the nitrogen-containing ones is well documented [1e, 6h].
The discovery of FSA and the structurally related transaldolase B mutant F178Y
(TalB F178Y ) has given a completely new dimension to the field. This is because
the possibility to use unphosphorylated DHA and analogs overcame the stringent
nucleophile specificity for the phosphorylated substrate that is typical for DHAP-
dependent aldolases. Hence, the improved structural diversity that can offer
variations of both donor and acceptor, in addition to the elimination of the
extra effort required to handle the phosphate group, makes FSA and TalB F178Y
biocatalysts for aldol addition with a tremendous prospect.
A number of different aldehydes have been utilized as acceptors, which in com-
bination with GO, HA, DHA, and HB donors yielded a plethora of structurally