Page 118 - Biodegradable Polyesters
P. 118
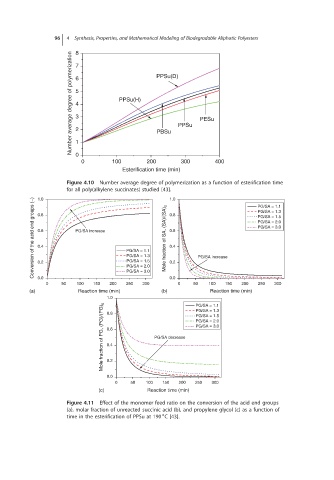
96 4 Synthesis, Properties, and Mathematical Modeling of Biodegradable Aliphatic Polyesters
8
Number average degree of polymerization 6 5 4 3 2 PPSu(H) PPSu(D) PPSu PESu
7
PBSu
1
0
0 100 200 300 400
Esterification time (min)
Figure 4.10 Number average degree of polymerization as a function of esterification time
for all poly(alkylene succinates) studied [43]. 1.0 PG/SA = 1.1
Conversion of the acid end groups (−) 0.8 PG/SA increase PG/SA = 1.1 Mole fraction of SA, (SA)/(SA) 0 0.8 PG/SA increase PG/SA = 1.5
1.0
PG/SA = 1.3
PG/SA = 2.0
PG/SA = 3.0
0.6
0.6
0.4
0.4
PG/SA = 1.3
PG/SA = 1.5
0.2
PG/SA = 3.0
0.0
0.0
0 50 100 150 200 PG/SA = 2.0 0.2 0 50 100 150 200 250 300
300
250
(a) Reaction time (min) (b) Reaction time (min)
1.0 PG/SA = 1.1
Mole fraction of PG, (PG)/(PG) 0 0.6 PG/SA decrease PG/SA = 2.0
PG/SA = 1.3
0.8
PG/SA = 1.5
PG/SA = 3.0
0.4
0.2
0.0
0 50 100 150 200 250 300
(c) Reaction time (min)
Figure 4.11 Effect of the monomer feed ratio on the conversion of the acid end groups
(a), molar fraction of unreacted succinic acid (b), and propylene glycol (c) as a function of
∘
time in the esterification of PPSu at 190 C [43].