Page 121 - Biodegradable Polyesters
P. 121
4.4 Mathematical Modeling of the Synthesis of Aliphatic Polyesters 99
denote the total amount of water which can be adsorbed on the particles and y
the instantaneous actual amount of adsorbed water divided by Ψ.Thedynamics
of y is described from the following balance including adsorption and desorption
kinetics [47]:
dy ( ) 1 (W) f 1
= k ads 1 − y ν − k des y ν (4.35)
dt V
3
−1
where k ads is the adsorption constant (m mol −1 −1 des (s )isthe desorp-
s )and k
tion constant. The total amount of water in the mixture (W) and the accumulated
(experimentally measured) H are estimated from
w
(W) = (W) + yΨ (4.36)
f
H = (S) − (S) + (W) (4.37)
0
w
Finally, the free water balance is written as
d(W) f =− d (S) −Ψ dy − (W)
dt dt dt f (4.38)
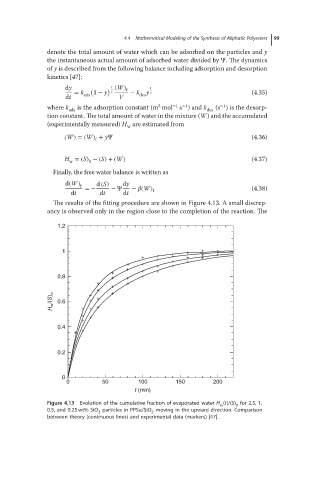
The results of the fitting procedure are shown in Figure 4.13. A small discrep-
ancy is observed only in the region close to the completion of the reaction. The
1.2
1
0.8
H w /(S) o 0.6
0.4
0.2
0
0 50 100 150 200
t (min)
Figure 4.13 Evolution of the cumulative fraction of evaporated water H (t)/(S) for 2.5, 1,
w 0
0.5, and 0.25 wt% SiO particles in PPSu/SiO moving in the upward direction. Comparison
2 2
between theory (continuous lines) and experimental data (markers) [47].