Page 119 - Biodegradable Polyesters
P. 119
4.4 Mathematical Modeling of the Synthesis of Aliphatic Polyesters 97
Equation 4.11a and mole fraction of the unreacted succinic acid Equation 4.11b
and propylene glycol Equation 4.11c varies with reaction time at five different ini-
tial monomer feed ratios (PG∕SA = 1.1, 1.3, 1.5, 2.0, and 3.0). It is observed that
as the PG/SA ratio is increased, SA is completely consumed at shorter reaction
times, while a large amount of PG remains unreacted. Moreover, as the PG/SA
ratio increases, the conversion of total acid end groups gradually increases, as
was reported for similar systems (e.g., transesterification of dimethyl terephthalate
with ethylene glycol [53]). This indicates that more PG is available for the esteri-
fication reactions. It has also been shown that the higher the ratio, the higher the
final conversion reached. However, more PG hinders the polycondensation reac-
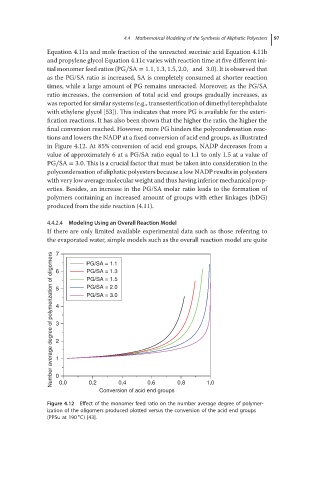
tions and lowers the NADP at a fixed conversion of acid end groups, as illustrated
in Figure 4.12. At 85% conversion of acid end groups, NADP decreases from a
value of approximately 6 at a PG/SA ratio equal to 1.1 to only 1.5 at a value of
PG∕SA = 3.0. This is a crucial factor that must be taken into consideration in the
polycondensation of aliphatic polyesters because a low NADP results in polyesters
with very low average molecular weight and thus having inferior mechanical prop-
erties. Besides, an increase in the PG/SA molar ratio leads to the formation of
polymers containing an increased amount of groups with ether linkages (bDG)
produced from the side reaction (4.11).
4.4.2.4 Modeling Using an Overall Reaction Model
If there are only limited available experimental data such as those referring to
the evaporated water, simple models such as the overall reaction model are quite
Number average degree of polymerization of oligomers
7
PG/SA = 1.1
6 PG/SA = 1.3
PG/SA = 1.5
5 PG/SA = 2.0
PG/SA = 3.0
4
3
2
1
0
0,0 0,2 0,4 0,6 0,8 1,0
Conversion of acid end groups
Figure 4.12 Effect of the monomer feed ratio on the number average degree of polymer-
ization of the oligomers produced plotted versus the conversion of the acid end groups
∘
(PPSu at 190 C) [43].