Page 142 - Biodegradable Polyesters
P. 142
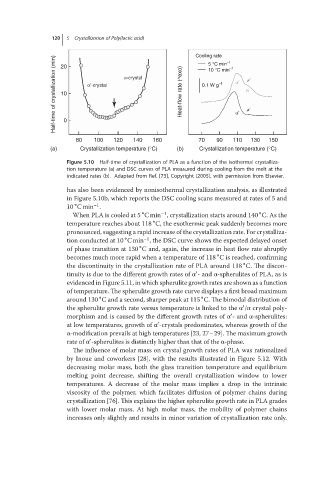
120 20 5 Crystallization of Poly(lactic acid) Cooling rate –1
Half-time of crystallization (min) 10 α′-crystal α-crystal Heat-flow rate (^exo) 0.1 W g –1 –1 α′ α
5 °C min
10 °C min
α′
0
80 100 120 140 160 70 90 110 130 150
(a) Crystallization temperature (°C) (b) Crystallization temperature (°C)
Figure 5.10 Half-time of crystallization of PLA as a function of the isothermal crystalliza-
tion temperature (a) and DSC curves of PLA measured during cooling from the melt at the
indicated rates (b). Adapted from Ref. [73], Copyright (2005), with permission from Elsevier.
has also been evidenced by nonisothermal crystallization analysis, as illustrated
in Figure 5.10b, which reports the DSC cooling scans measured at rates of 5 and
∘
−1
10 Cmin .
∘ ∘
−1
When PLA is cooled at 5 Cmin , crystallization starts around 140 C. As the
∘
temperature reaches about 118 C, the exothermic peak suddenly becomes more
pronounced, suggesting a rapid increase of the crystallization rate. For crystalliza-
∘
−1
tion conducted at 10 Cmin , the DSC curve shows the expected delayed onset
∘
of phase transition at 130 C and, again, the increase in heat flow rate abruptly
∘
becomes much more rapid when a temperature of 118 C is reached, confirming
∘
the discontinuity in the crystallization rate of PLA around 118 C. The discon-
′
tinuity is due to the different growth rates of α -and α-spherulites of PLA, as is
evidenced in Figure 5.11, in which spherulite growth rates are shown as a function
of temperature. The spherulite growth rate curve displays a first broad maximum
∘
∘
around 130 C and a second, sharper peak at 115 C. The bimodal distribution of
′
the spherulite growth rate versus temperature is linked to the α /α crystal poly-
′
morphism and is caused by the different growth rates of α -and α-spherulites:
′
at low temperatures, growth of α -crystals predominates, whereas growth of the
α-modification prevails at high temperatures [23, 27–29]. The maximum growth
′
rate of α -spherulites is distinctly higher than that of the α-phase.
The influence of molar mass on crystal growth rates of PLA was rationalized
by Inoue and coworkers [28], with the results illustrated in Figure 5.12. With
decreasing molar mass, both the glass transition temperature and equilibrium
melting point decrease, shifting the overall crystallization window to lower
temperatures. A decrease of the molar mass implies a drop in the intrinsic
viscosity of the polymer, which facilitates diffusion of polymer chains during
crystallization [76]. This explains the higher spherulite growth rate in PLA grades
with lower molar mass. At high molar mass, the mobility of polymer chains
increases only slightly and results in minor variation of crystallization rate only.