Page 137 - Biodegradable Polyesters
P. 137
5.3 Kinetics of Crystal Nucleation 115
Li (2008)
10 4 Yasuniwa (2006)
Tsuji (2006)
Tsuji (1996)
10 3
Density 10 2
10 1
10 0
90 100 110 120 130 140 150
Crystallization temperature (°C)
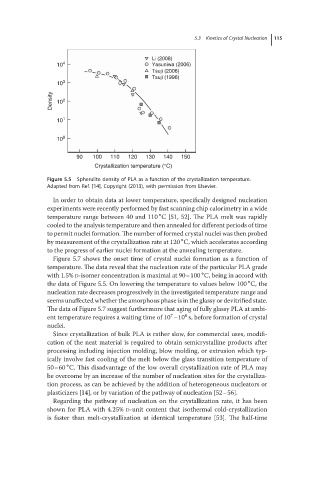
Figure 5.5 Spherulite density of PLA as a function of the crystallization temperature.
Adapted from Ref. [14], Copyright (2013), with permission from Elsevier.
In order to obtain data at lower temperature, specifically designed nucleation
experiments were recently performed by fast scanning chip calorimetry in a wide
∘
temperature range between 40 and 110 C [51, 52]. The PLA melt was rapidly
cooled to the analysis temperature and then annealed for different periods of time
to permit nuclei formation. The number of formed crystal nuclei was then probed
∘
by measurement of the crystallization rate at 120 C, which accelerates according
to the progress of earlier nuclei formation at the annealing temperature.
Figure 5.7 shows the onset time of crystal nuclei formation as a function of
temperature. The data reveal that the nucleation rate of the particular PLA grade
∘
with 1.5% D-isomer concentration is maximal at 90–100 C, being in accord with
∘
the data of Figure 5.5. On lowering the temperature to values below 100 C, the
nucleation rate decreases progressively in the investigated temperature range and
seems unaffected whether the amorphous phase is in the glassy or devitrified state.
The data of Figure 5.7 suggest furthermore that aging of fully glassy PLA at ambi-
7
8
ent temperature requires a waiting time of 10 –10 s, before formation of crystal
nuclei.
Since crystallization of bulk PLA is rather slow, for commercial uses, modifi-
cation of the neat material is required to obtain semicrystalline products after
processing including injection molding, blow molding, or extrusion which typ-
ically involve fast cooling of the melt below the glass transition temperature of
∘
50–60 C. This disadvantage of the low overall crystallization rate of PLA may
be overcome by an increase of the number of nucleation sites for the crystalliza-
tion process, as can be achieved by the addition of heterogeneous nucleators or
plasticizers [14], or by variation of the pathway of nucleation [52–56].
Regarding the pathway of nucleation on the crystallization rate, it has been
shown for PLA with 4.25% D-unit content that isothermal cold-crystallization
is faster than melt-crystallization at identical temperature [53]. The half-time