Page 140 - Biodegradable Polyesters
P. 140
118 5 Crystallization of Poly(lactic acid)
50 °C 55 °C 60 °C 65 °C 70 °C
2 min
10 min
30 min
100 min
Crystallization
120 °C, 10 min
T
500 min g
Annealing
Temperature RT
Image capture
1000 min Time
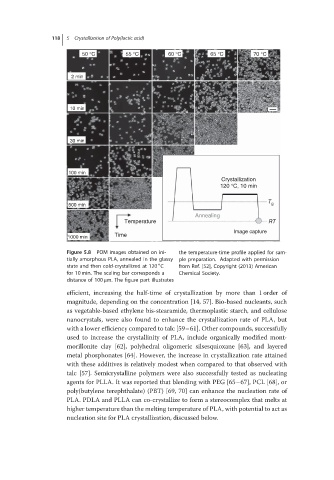
Figure 5.8 POMimagesobtainedonini- the temperature-time profile applied for sam-
tially amorphous PLA, annealed in the glassy ple preparation. Adapted with permission
∘
state and then cold-crystallized at 120 C from Ref. [52], Copyright (2013) American
for 10 min. The scaling bar corresponds a Chemical Society.
distance of 100 μm. The figure part illustrates
efficient, increasing the half-time of crystallization by more than 1 order of
magnitude, depending on the concentration [14, 57]. Bio-based nucleants, such
as vegetable-based ethylene bis-stearamide, thermoplastic starch, and cellulose
nanocrystals, were also found to enhance the crystallization rate of PLA, but
with a lower efficiency compared to talc [59–61]. Other compounds, successfully
used to increase the crystallinity of PLA, include organically modified mont-
morillonite clay [62], polyhedral oligomeric silsesquioxane [63], and layered
metal phosphonates [64]. However, the increase in crystallization rate attained
with these additives is relatively modest when compared to that observed with
talc [57]. Semicrystalline polymers were also successfully tested as nucleating
agents for PLLA. It was reported that blending with PEG [65–67], PCL [68], or
poly(butylene terephthalate) (PBT) [69, 70] can enhance the nucleation rate of
PLA. PDLA and PLLA can co-crystallize to form a stereocomplex that melts at
higher temperature than the melting temperature of PLA, with potential to act as
nucleation site for PLA crystallization, discussed below.