Page 132 - Biodegradable Polyesters
P. 132
110 5 Crystallization of Poly(lactic acid)
reaction [9, 10]. This led to large-scale use of this bio-based polymer, transforming
PLA from a specialty material to a commodity thermoplastic.
The increased availability of PLA stimulated an increase in its research and
development activities. A survey of the literature revealed that the number of pub-
lished articles related to PLA has increased exponentially over the past decade,
and about 1500 new research papers have been published yearly in recent years
[11]. This can be also partly attributed to the growing environmental concern that
stimulates the use of bio-based polymers.
PLA can be synthesized by two routes: polycondensation of lactic acid or ring-
opening polymerization of its cyclic dimer, lactide [12]. PLA prepared from poly-
condensation has low molar mass and poor mechanical properties and is therefore
not suitable for many applications [13]. High-molar-mass PLA is most commonly
made by ring-opening polymerization of lactide. In both cases, lactic acid is the
feedstock for PLA production. Lactic acid has an asymmetric carbon atom, which
leads to two optically active forms called L-lactic acid and D-lactic acid. When pro-
ducing PLA from lactide, polymerization can start from three types of monomers:
LL-lactide made from two L-lactic acid molecules, DD-lactide from dimerization of
D-lactic acid, and LD or meso-lactide made from a combination of one L-and one
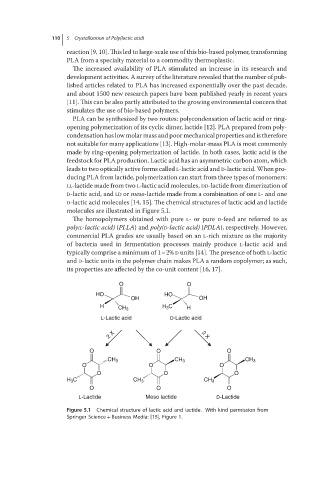
D-lactic acid molecules [14, 15]. The chemical structures of lactic acid and lactide
molecules are illustrated in Figure 5.1.
The homopolymers obtained with pure L- or pure D-feed are referred to as
poly(L-lactic acid) (PLLA)and poly(D-lactic acid) (PDLA), respectively. However,
commercial PLA grades are usually based on an L-rich mixture as the majority
of bacteria used in fermentation processes mainly produce L-lactic acid and
typically comprise a minimum of 1–2% D units [14]. The presence of both L-lactic
and D-lactic units in the polymer chain makes PLA a random copolymer; as such,
its properties are affected by the co-unit content [16, 17].
O O
HO HO
OH OH
H H 3 C
CH 3 H
L-Lactic acid D-Lactic acid
2 X 2 X
O O O
CH 3 CH 3 CH 3
O O O
O O O
H 3 C CH 3 CH 3
O O O
L-Lactide Meso lactide D-Lactide
Figure 5.1 Chemical structure of lactic acid and lactide. With kind permission from
Springer Science + Business Media: [15], Figure 1.