Page 35 - Biodegradable Polyesters
P. 35
1.3 Biodegradable Polyesters 13
O
O
O Ring-opening
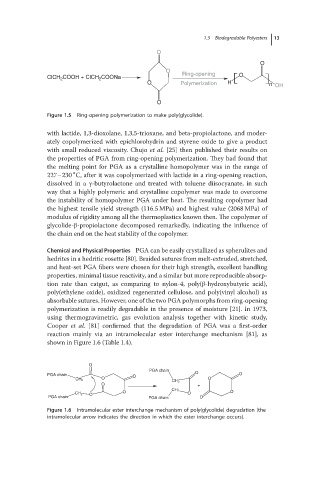
ClCH 2 COOH + ClCH 2 COONa O
O Polymerization H n OH
O
Figure 1.5 Ring-opening polymerization to make poly(glycolide).
with lactide, 1,3-dioxolane, 1,3,5-trioxane, and beta-propiolactone, and moder-
ately copolymerized with epichlorohydrin and styrene oxide to give a product
with small reduced viscosity. Chujo et al. [25] then published their results on
the properties of PGA from ring-opening polymerization. They had found that
the melting point for PGA as a crystalline homopolymer was in the range of
∘
227–230 C, after it was copolymerized with lactide in a ring-opening reaction,
dissolved in a γ-butyrolactone and treated with toluene diisocyanate, in such
way that a highly polymeric and crystalline copolymer was made to overcome
the instability of homopolymer PGA under heat. The resulting copolymer had
the highest tensile yield strength (116.5 MPa) and highest value (2068 MPa) of
modulus of rigidity among all the thermoplastics known then. The copolymer of
glycolide-β-propiolactone decomposed remarkedly, indicating the influence of
the chain end on the heat stability of the copolymer.
Chemical and Physical Properties PGA can be easily crystallized as spherulites and
hedrites in a hedritic rosette [80]. Braided sutures from melt-extruded, stretched,
and heat-set PGA fibers were chosen for their high strength, excellent handling
properties, minimal tissue reactivity, and a similar but more reproducible absorp-
tion rate than catgut, as comparing to nylon-4, poly(β-hydroxybutyric acid),
poly(ethylene oxide), oxidized regenerated cellulose, and poly(vinyl alcohol) as
absorbable sutures. However, one of the two PGA polymorphs from ring-opening
polymerization is readily degradable in the presence of moisture [21]. In 1973,
using thermogravimetric, gas evolution analysis together with kinetic study,
Cooper et al. [81] confirmed that the degradation of PGA was a first-order
reaction mainly via an intramolecular ester interchange mechanism [81], as
shown in Figure 1.6 (Table 1.4).
O
PGA chain
PGA chain O O O
O O
CH 2
CH 2
O +
O CH 2 O
CH 2 O O
PGA chain PGA chain O
Figure 1.6 Intramolecular ester interchange mechanism of poly(glycolide) degradation (the
intramolecular arrow indicates the direction in which the ester interchange occurs).