Page 32 - Biodegradable Polyesters
P. 32
10 1 Biodegradable Polyesters: Synthesis, Properties, Applications
2) Lactic acid can be polymerized in solution to produce high-molecular-weight
PLA.
3) PLA at low DP depolymerizes to lactide either through ring-opening poly-
merization with stannous octoate as catalyst and SnCl or through copoly-
2
merization with comonomers in solution to obtain high-molecular-weight
PLA copolymers [24]. In this way, the reaction does not generate additional
water and hence, a wide range of molecular weights is obtainable;
4) Lactic acid reacts with diacid or diol to form telechelic polylactic acid, then
through further a linking reaction it forms high-molecular-weight lactic
acid copolymers [33, 44, 45]. Polymerization of a racemic mixture of L-and
D-lactides usually leads to the synthesis of poly-DL-lactide (PDLLA)which
is amorphous. Use of stereospecific catalysts can result in heterotactic PLA
which is found to show crystallinity [46, 47]. The degree of crystallinity
and many associated properties are greatly controlled by the ratio of D to L
enantiomers in the polymer [48].
Chemical and Physical Properties Owing to the chirality of lactic acid, different
forms of polylactide exist as PLLA [49], poly-D-lactide (PDLA) [50], PDLLA [51],
and poly(L-lactide-co-D,L-lactide) (PLDLLA) [52]. Poly(lactide)s such as PLA and
lactide copolymers are biodegradable and nontoxic to the human body and the
environment. They have been used as biomedical materials for tissue regeneration,
matrices for drug delivery systems, and alternatives for commercial polymeric
materials to minimize the impact on the environment. With stereocomplex for-
mation between enantiomeric PLA, numerous studies have been carried out with
respect to the formation, structure, properties, degradation, and applications of
the PLA stereocomplexes. Stereocomplexation enhances the mechanical proper-
ties, the thermal resistance, and the hydrolysis-resistance of PLA-based materials.
These improvements arise from a peculiarly strong interaction between L-lactyl
unit sequences and D-lactyl unit sequences. Stereocomplexation opens a new way
for the preparation of biomaterials such as hydrogels and particles for drug deliv-
ery systems. PLA stereocomplexation, and the structure, properties, degradation,
and applications of a variety of stereocomplexed PLA materials have been studied
[32, 50–58] (Table 1.3).
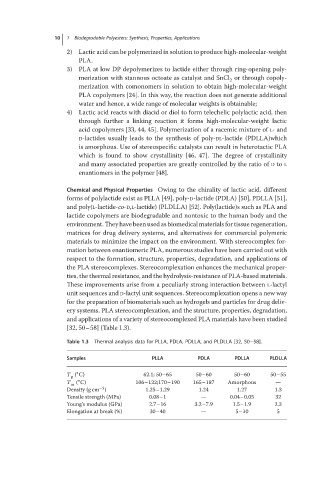
Table 1.3 Thermal analysis data for PLLA, PDLA, PDLLA, and PLDLLA [32, 50–58].
Samples PLLA PDLA PDLLA PLDLLA
∘
T ( C) 62.1; 50–65 50–60 50–60 50–55
g
∘
T m ( C) 106–122;170–190 165–187 Amorphous —
−3
Density (g cm ) 1.25–1.29 1.24 1.27 1.3
Tensile strength (MPa) 0.08–1 — 0.04–0.05 32
Young’s modulus (GPa) 2.7–16 3.2–7.9 1.5–1.9 2.3
Elongation at break (%) 30–40 — 5–10 5