Page 243 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 243
220 Biomass Gasification, Pyrolysis and Torrefaction
7.4.1.2 Gibbs Free Energy
Gibbs free energy, G, is an important thermodynamic function. Its change in
terms of a change in entropy, ΔS, and enthalpy, ΔH, is written as:
ΔG 5 ΔH 2 TΔS (7.34)
The change in enthalpy or entropy for a reaction system is computed by
finding the enthalpy or entropy changes of individual gases in the system. It
is explained in Example 7.2. An alternative approach uses the empirical
equations given by Probstein and Hicks (2006). It expresses the Gibbs func-
tion (Eq. (7.35)) and the enthalpy of formation (Eq. (7.36)) in terms of tem-
perature, T, the heat of formation at the reference state at 1 atm and 298 K,
0
0
and a number of empirical coefficients, a , b , and so forth.
c 0 3 d 0 4
0
0
0 2
ΔG 5 Δh 2 a T lnðTÞ 2 b T 2 T 2 T
0
f;T 298
2 3
(7.35)
e
0
1 1 f 1 g T kJ=mol
0
0
2T
e 0
0 2
0 3
0 4
0
0
0
0
ΔH f;T 5 Δh 298 2 a T 1 b T 1 c T 1 d T 1 1 f kJ=mol (7.36)
T
The values of the empirical coefficients for some common gases are given
in Table 7.5.
The equilibrium constant of a reaction occurring at a temperature T may
be known using the value of Gibbs free energy.
ΔG
K e 5 exp 2 (7.37)
RT
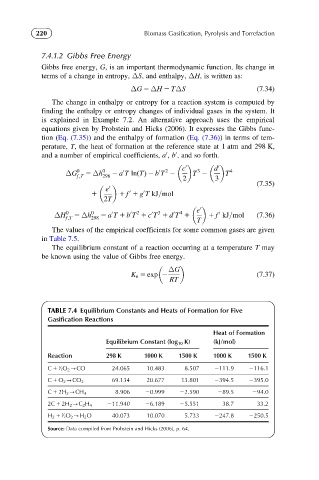
TABLE 7.4 Equilibrium Constants and Heats of Formation for Five
Gasification Reactions
Heat of Formation
Equilibrium Constant (log 10 K) (kJ/mol)
Reaction 298 K 1000 K 1500 K 1000 K 1500 K
1
C 1 /2O 2 -CO 24.065 10.483 8.507 2111.9 2116.1
C 1 O 2 -CO 2 69.134 20.677 13.801 2394.5 2395.0
C 1 2H 2 -CH 4 8.906 20.999 22.590 289.5 294.0
2C 1 2H 2 -C 2 H 4 211.940 26.189 25.551 38.7 33.2
1
H 2 1 /2O 2 -H 2 O 40.073 10.070 5.733 2247.8 2250.5
Source: Data compiled from Probstein and Hicks (2006), p. 64.