Page 244 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 244
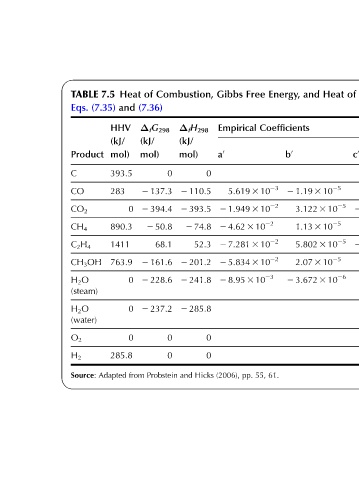
of from Coefficients Empirical and atm, 1 K, 298 at Formation g 0 f 0 e 0 d 0 c 0 2 6.131 3 10 22 0.868 2 4.891 3 10 2 2 1.846 3 10 212 6.383 3 10 29 2 0.1207 5.27 2 4.891 3 10 2 6.946 3 10 212 2 2.448 3 10 28 0.2234 14.11 2 4.891 3 10 2 2 6.647 3 10 212 1.319 3 10 28 2 0.4076 20.32 2 9.782 3 10 2 5.648
Heat
and 2 1.19 3 10 25 3.122 3 10 25 1.13 3 10 25 5.802 3 10 25 2.07 3 10 25 2 3.672 3 10 26
Energy, Coefficients b 0 61.
Free 5.619 3 10 23 2 1.949 3 10 22 2 4.62 3 10 22 2 7.281 3 10 22 2 5.834 3 10 22 2 8.95 3 10 23 55, pp.
Gibbs Empirical a 0 0 0 0 (2006),
Combustion, Δ f H 298 (kJ/ mol) 0 2 110.5 2 393.5 2 74.8 52.3 2 201.2 2 241.8 2 285.8 0 0 Hicks and
of (7.36) Δ f G 298 (kJ/ mol) 2 137.3 2 394.4 2 50.8 68.1 2 161.6 2 228.6 2 237.2 Probstein
Heat and HHV mol) 393.5 0 890.3 1411 763.9 0 0 0 285.8 from
7.5 (7.35) (kJ/ 283 Adapted
TABLE Eqs. Product C CO CO 2 CH 4 C 2 H 4 CH 3 OH H 2 O (steam) H 2 O (water) O 2 H 2 Source: