Page 248 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 248
224 Biomass Gasification, Pyrolysis and Torrefaction
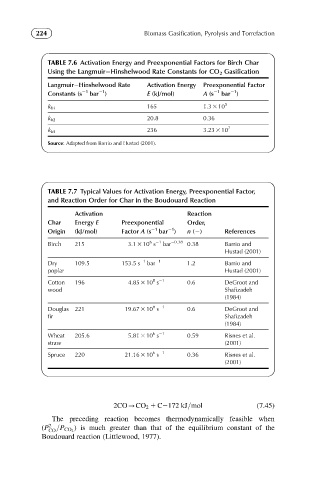
TABLE 7.6 Activation Energy and Preexponential Factors for Birch Char
Using the Langmuir Hinshelwood Rate Constants for CO 2 Gasification
Langmuir Hinshelwood Rate Activation Energy Preexponential Factor
21
21
Constants (s 21 bar ) E (kJ/mol) A (s 21 bar )
165 1.3 3 10 5
k b1
20.8 0.36
k b2
k b3 236 3.23 3 10 7
Source: Adapted from Barrio and Hustad (2001).
TABLE 7.7 Typical Values for Activation Energy, Preexponential Factor,
and Reaction Order for Char in the Boudouard Reaction
Activation Reaction
Char Energy E Preexponential Order,
21
Origin (kJ/mol) Factor A (s 21 bar ) n (2) References
6 21
Birch 215 3.1 3 10 s bar 20.38 0.38 Barrio and
Hustad (2001)
Dry 109.5 153.5 s 21 bar 21 1.2 Barrio and
poplar Hustad (2001)
8 21
Cotton 196 4.85 3 10 s 0.6 DeGroot and
wood Shafizadeh
(1984)
8 21
Douglas 221 19.67 3 10 s 0.6 DeGroot and
fir Shafizadeh
(1984)
6 21
Wheat 205.6 5.81 3 10 s 0.59 Risnes et al.
straw (2001)
6 21
Spruce 220 21.16 3 10 s 0.36 Risnes et al.
(2001)
2CO-CO 2 1 C2172 kJ=mol (7.45)
The preceding reaction becomes thermodynamically feasible when
(P 2 CO =P CO 2 ) is much greater than that of the equilibrium constant of the
Boudouard reaction (Littlewood, 1977).