Page 245 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 245
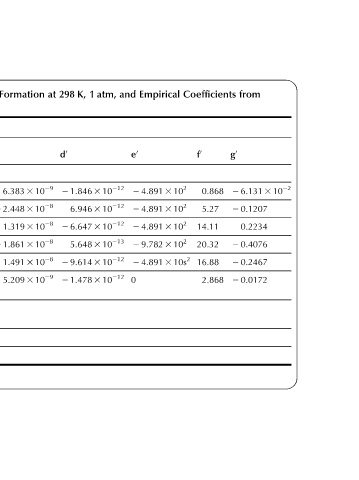
2 6.131 3 10 22
from g 0 2 0.1207 0.2234 2 0.4076 2 0.2467 2 0.0172
Coefficients f 0 0.868 5.27 14.11 20.32 16.88 2.868
Empirical 2 4.891 3 10 2 2 4.891 3 10 2 2 4.891 3 10 2 2 9.782 3 10 2 2 4.891 3 10s 2
and e 0 0
atm,
1 2 1.846 3 10 212 6.946 3 10 212 2 6.647 3 10 212 5.648 3 10 213 2 9.614 3 10 212 2 1.478 3 10 212
K,
298 d 0
at
Formation 6.383 3 10 29 2 2.448 3 10 28 1.319 3 10 28 2 1.861 3 10 28 1.491 3 10 28 5.209 3 10 29
of c 0
Heat
and 2 1.19 3 10 25 3.122 3 10 25 1.13 3 10 25 5.802 3 10 25 2.07 3 10 25 2 3.672 3 10 26
Energy, Coefficients b 0 61.
Free 5.619 3 10 23 2 1.949 3 10 22 2 4.62 3 10 22 2 7.281 3 10 22 2 5.834 3 10 22 2 8.95 3 10 23 55, pp.
Gibbs Empirical a 0 0 0 0 (2006),
Combustion, Δ f H 298 (kJ/ mol) 0 2 110.5 2 393.5 2 74.8 52.3 2 201.2 2 241.8 2 285.8 0 0 Hicks and
of (7.36) Δ f G 298 (kJ/ mol) 2 137.3 2 394.4 2 50.8 68.1 2 161.6 2 228.6 2 237.2 Probstein
Heat and HHV mol) 393.5 0 890.3 1411 763.9 0 0 0 285.8 from
7.5 (7.35) (kJ/ 283 Adapted
TABLE Eqs. Product C CO CO 2 CH 4 C 2 H 4 CH 3 OH H 2 O (steam) H 2 O (water) O 2 H 2 Source: