Page 32 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 32
Chapter | 1 Introduction 11
Pyrolysis involves rapid heating in the total absence of oxygen. In liquefac-
tion, the large molecules of solid feedstock are decomposed into liquids
having smaller molecules. This occurs in the presence of a catalyst and at a
still lower temperature.
Table 1.3 compares basic features of thermochemical and biochemical
routes for biomass conversion. It gives that the biochemical route for ethanol
production is commercially more developed than the thermochemical route, but
the former requires sugar or starch for feedstock; it cannot use more plentiful
lignocellulosic stuff. As a result, a larger fraction of the available biomass is
not converted into ethanol. For example, in a corn plant, only the kernel is uti-
lized for ethanol production. The stover, stalk, roots, and leaves, which consti-
tute bulk of the corn plant, are left as wastes as being lignocellulosic. Even
though the enzymatic or biochemical route is more developed, this is a batch
process and takes an order of magnitude longer to complete than the thermo-
chemical process.
In the thermochemical route (Figure 1.5), the biomass is first converted
into syngas, which is then converted into ethanol through synthesis or some
other means.
1.2.2.1 Combustion
Combustion represents perhaps the oldest means of utilization of biomass,
given that civilization began with the discovery of fire. The burning of forest
wood taught humans how to cook and how to keep themselves warm.
Chemically, combustion is an exothermic reaction between oxygen and
hydrocarbon in biomass. Here, the biomass is oxidized into two major
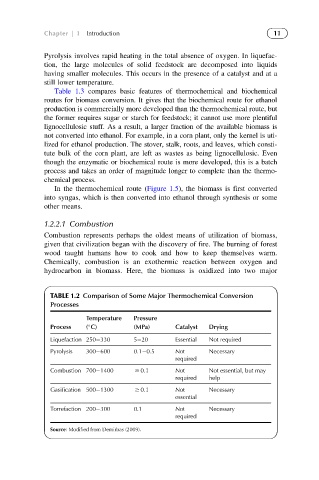
TABLE 1.2 Comparison of Some Major Thermochemical Conversion
Processes
Temperature Pressure
Process ( C) (MPa) Catalyst Drying
Liquefaction 250 330 5 20 Essential Not required
Pyrolysis 300 600 0.1 0.5 Not Necessary
required
Combustion 700 1400 $ 0.1 Not Not essential, but may
required help
Gasification 500 1300 $ 0.1 Not Necessary
essential
Torrefaction 200 300 0.1 Not Necessary
required
Source: Modified from Demirbas (2009).