Page 35 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 35
14 Biomass Gasification, Pyrolysis, and Torrefaction
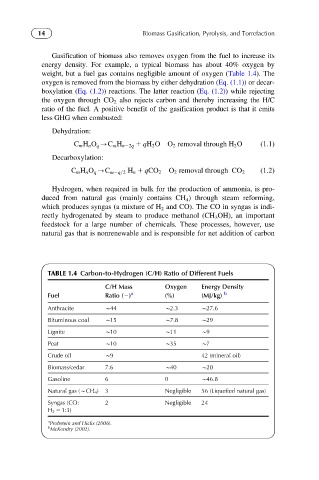
Gasification of biomass also removes oxygen from the fuel to increase its
energy density. For example, a typical biomass has about 40% oxygen by
weight, but a fuel gas contains negligible amount of oxygen (Table 1.4). The
oxygen is removed from the biomass by either dehydration (Eq. (1.1))ordecar-
boxylation (Eq. (1.2)) reactions. The latter reaction (Eq. (1.2)) while rejecting
the oxygen through CO 2 also rejects carbon and thereby increasing the H/C
ratio of the fuel. A positive benefit of the gasification product is that it emits
less GHG when combusted:
Dehydration:
C m H n O q -C m H n22q 1 qH 2 O O 2 removal through H 2 O (1.1)
Decarboxylation:
(1.2)
C m H n O q -C m2q=2 H n 1 qCO 2 O 2 removal through CO 2
Hydrogen, when required in bulk for the production of ammonia, is pro-
duced from natural gas (mainly contains CH 4 ) through steam reforming,
which produces syngas (a mixture of H 2 and CO). The CO in syngas is indi-
rectly hydrogenated by steam to produce methanol (CH 3 OH), an important
feedstock for a large number of chemicals. These processes, however, use
natural gas that is nonrenewable and is responsible for net addition of carbon
TABLE 1.4 Carbon-to-Hydrogen (C/H) Ratio of Different Fuels
C/H Mass Oxygen Energy Density
Fuel Ratio (2) a (%) (MJ/kg) b
Anthracite B44 B2.3 B27.6
Bituminous coal B15 B7.8 B29
Lignite B10 B11 B9
Peat B10 B35 B7
Crude oil B9 42 (mineral oil)
Biomass/cedar 7.6 B40 B20
Gasoline 6 0 B46.8
Natural gas (BCH 4 ) 3 Negligible 56 (Liquefied natural gas)
Syngas (CO: 2 Negligible 24
H 2 5 1:3)
a Probstein and Hicks (2006).
b
McKendry (2002).