Page 345 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 345
322 BIOMATERIALS
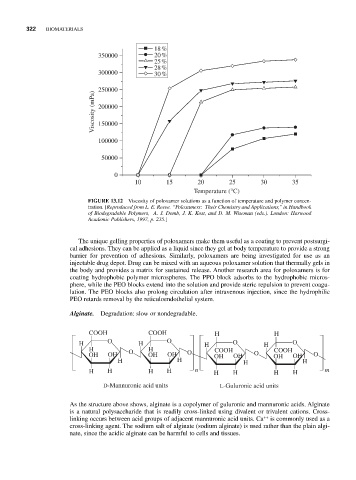
18 %
350000 20 %
25 %
28 %
300000 30 %
250000
Viscosity (mPa) 200000
150000
100000
50000
0
10 15 20 25 30 35
Temperature (°C)
FIGURE 13.12 Viscosity of poloxamer solutions as a function of temperature and polymer concen-
tration. [Reproduced from L. E. Reeve. “Poloxamers: Their Chemistry and Applications,” in Handbook
of Biodegradable Polymers, A. J. Domb, J. K. Kost, and D. M. Wiseman (eds.). London: Harwood
Academic Publishers, 1997, p. 235.]
The unique gelling properties of poloxamers make them useful as a coating to prevent postsurgi-
cal adhesions. They can be applied as a liquid since they gel at body temperature to provide a strong
barrier for prevention of adhesions. Similarly, poloxamers are being investigated for use as an
injectable drug depot. Drug can be mixed with an aqueous poloxamer solution that thermally gels in
the body and provides a matrix for sustained release. Another research area for poloxamers is for
coating hydrophobic polymer microspheres. The PPO block adsorbs to the hydrophobic micros-
phere, while the PEO blocks extend into the solution and provide steric repulsion to prevent coagu-
lation. The PEO blocks also prolong circulation after intravenous injection, since the hydrophilic
PEO retards removal by the reticuloendothelial system.
Alginate. Degradation: slow or nondegradable.
COOH COOH H H
O O O O
H H H H
H H COOH COOH
OH OH O OH OH O OH OH O OH OH O
H H H H
H H H H n H H H H m
D-Mannuronic acid units L-Guluronic acid units
As the structure above shows, alginate is a copolymer of guluronic and mannuronic acids. Alginate
is a natural polysaccharide that is readily cross-linked using divalent or trivalent cations. Cross-
linking occurs between acid groups of adjacent mannuronic acid units. Ca ++ is commonly used as a
cross-linking agent. The sodium salt of alginate (sodium alginate) is used rather than the plain algi-
nate, since the acidic alginate can be harmful to cells and tissues.