Page 387 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 387
364 BIOMATERIALS
motion at the implant/tissue interface (Ducheyne et al., 1987). For resorbable materials, additional
design requirements include: the need to maintain strength and stability of the material/tissue
interface during material degradation and host tissue regeneration; material resorption and tissue
repair/regeneration rates should be matched; and the resorbable material should consist only of meta-
bolically acceptable species.
15.3.1 Bioactive Glasses and Glass Ceramics
Bioactive glasses are used as bulk implants, coatings on metal or ceramic implants, and scaffolds for
guiding biological therapies (Kohn and Ducheyne, 1992; Hench et al., 1972; El-Ghannam et al., 1997;
Gross and Strunz, 1980; Nakamura et al., 1985; Radin et al., 2005; Reilly et al., 2007) (Table 15.2).
Chemical reactions are limited to the surface (~300 to 500 μm) of the glass, and bulk properties are
not affected by surface reactivity. The degree of activity and physiologic response are dependent on
the chemical composition of the glass, and may vary by over an order of magnitude. For example,
the substitution of CaF for CaO decreases solubility, while addition of B O increases solubility
2
3
(Hench and Ethridge, 1982).
®
Ceravital, a variation of Bioglass , is a glass ceramic. The seed material is quench-melted to form
a glass, then heat-treated to form nuclei for crystal growth and transformation from a glass to a
®
ceramic. Ceravital has a different alkali oxide concentration than Bioglass —small amounts of alka-
line oxides are added to control dissolution rates (Table 15.2)—but the physiological response to
both glasses is similar (Gross and Strunz, 1980). A glass ceramic containing crystalline oxyapatite,
fluorapatite, and β-Wollastonite in a glassy matrix, denoted glass-ceramic A-W, is another bioactive
glass ceramic (Kitsugi et al., 1986; Kokubo et al., 1990a, 1990b; Nakamura et al., 1985). A-W glass-
ceramic bonds to bone through a thin calcium and phosphorus-rich layer, which is formed at the sur-
face of the glass ceramic (Kitsugi et al., 1986; Nakamura et al., 1985). In vitro, if the physiological
environment is correctly simulated in terms of ion concentration, pH, and temperature, this layer con-
sists of small carbonated hydroxyapatite (HA) crystallites with a defective structure, and the com-
position and structural characteristics are similar to those of bone (Kokubo et al., 1990a).
Glass and glass-ceramics interface with the biological milieu because ceramics are susceptible to
surface changes in an aqueous media. Lower valence ions segregate to surfaces and grain boundaries,
leading to concentration gradients and ion exchange. These reactions are dependent on the local pH and
reactive cellular constituents (Hench and Ethridge, 1982), and can be biologically beneficial or adverse.
Therefore, the surface reactions of glass ceramics should be well-controlled and characterized.
+
+
When placed in physiological media, bioactive glasses leach Na ions, and subsequently K ,
4+
2+
5+
+
Ca , P , Si , and Si-OH. These ionic species are replaced with H O ions from the media through
3
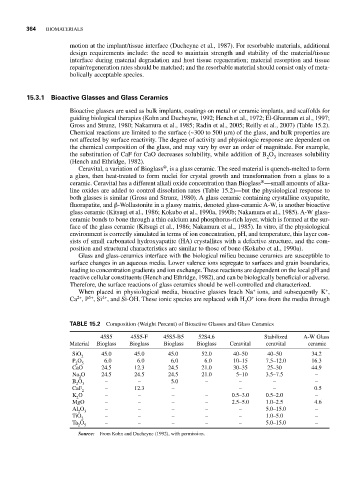
TABLE 15.2 Composition (Weight Percent) of Bioactive Glasses and Glass Ceramics
45S5 45S5-F 45S5-B5 52S4.6 Stabilized A-W Glass
Material Bioglass Bioglass Bioglass Bioglass Ceravital ceravital ceramic
SiO 45.0 45.0 45.0 52.0 40–50 40–50 34.2
2
P O 6.0 6.0 6.0 6.0 10–15 7.5–12.0 16.3
2 5
CaO 24.5 12.3 24.5 21.0 30–35 25–30 44.9
Na O 24.5 24.5 24.5 21.0 5–10 3.5–7.5 –
2
B O – – 5.0 – – – –
2 3
CaF – 12.3 – – – 0.5
2
K O – – – – 0.5–3.0 0.5–2.0 –
2
MgO – – – – 2.5–5.0 1.0–2.5 4.6
Al O – – – – – 5.0–15.0 –
2 3
TiO – – – – – 1.0–5.0 –
2
Ta O – – – – – 5.0–15.0 –
2 5
Source: From Kohn and Ducheyne (1992), with permission.