Page 389 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 389
366 BIOMATERIALS
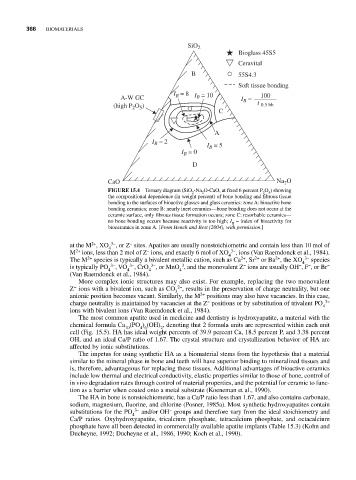
SiO 2
Bioglass 45S5
Ceravital
B 55S4.3
Soft tissue bonding
I B = 8 I = 10 100
A-W GC B I B =
(high P O ) t 0.5 bb
2 5
C
A
I = 2 I = 5
B
B
I = 0
B
D
CaO Na 2 O
FIGURE 15.4 Ternary diagram (SiO -Na O-CaO, at fixed 6 percent P O ) showing
2 2 2 5
the compositional dependence (in weight percent) of bone bonding and fibrous tissue
bonding to the surfaces of bioactive glasses and glass ceramics: zone A: bioactive bone
bonding ceramics; zone B: nearly inert ceramics—bone bonding does not occur at the
ceramic surface, only fibrous tissue formation occurs; zone C: resorbable ceramics—
no bone bonding occurs because reactivity is too high; I = index of bioactivity for
B
bioceramics in zone A. [From Hench and Best (2004), with permission.]
2+
−
at the M , XO 4 3− , or Z sites. Apatites are usually nonstoichiometric and contain less than 10 mol of
−
2+
M ions, less than 2 mol of Z ions, and exactly 6 mol of XO 4 3− , ions (Van Raemdonck et al., 1984).
2+
2+
2+
2+
The M species is typically a bivalent metallic cation, such as Ca , Sr or Ba , the XO 4 3− species
−
−
−
3
is typically PO 3− , VO 3− , CrO 3− , or MnO , and the monovalent Z ions are usually OH , F , or Br −
4 4 4 4
(Van Raemdonck et al., 1984).
More complex ionic structures may also exist. For example, replacing the two monovalent
−
Z ions with a bivalent ion, such as CO 2− , results in the preservation of charge neutrality, but one
3
2+
anionic position becomes vacant. Similarly, the M positions may also have vacancies. In this case,
−
charge neutrality is maintained by vacancies at the Z positions or by substitution of trivalent PO 3−
4
ions with bivalent ions (Van Raemdonck et al., 1984).
The most common apatite used in medicine and dentistry is hydroxyapatite, a material with the
chemical formula Ca (PO ) (OH) , denoting that 2 formula units are represented within each unit
10 4 6 2
cell (Fig. 15.5). HA has ideal weight percents of 39.9 percent Ca, 18.5 percent P, and 3.38 percent
OH, and an ideal Ca/P ratio of 1.67. The crystal structure and crystallization behavior of HA are
affected by ionic substitutions.
The impetus for using synthetic HA as a biomaterial stems from the hypothesis that a material
similar to the mineral phase in bone and teeth will have superior binding to mineralized tissues and
is, therefore, advantageous for replacing these tissues. Additional advantages of bioactive ceramics
include low thermal and electrical conductivity, elastic properties similar to those of bone, control of
in vivo degradation rates through control of material properties, and the potential for ceramic to func-
tion as a barrier when coated onto a metal substrate (Koeneman et al., 1990).
The HA in bone is nonstoichiometric, has a Ca/P ratio less than 1.67, and also contains carbonate,
sodium, magnesium, fluorine, and chlorine (Posner, 1985a). Most synthetic hydroxyapatites contain
−
substitutions for the PO 3− and/or OH groups and therefore vary from the ideal stoichiometry and
4
Ca/P ratios. Oxyhydroxyapatite, tricalcium phosphate, tetracalcium phosphate, and octacalcium
phosphate have all been detected in commercially available apatite implants (Table 15.3) (Kohn and
Ducheyne, 1992; Ducheyne et al., 1986, 1990; Koch et al., 1990).