Page 393 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 393
370 BIOMATERIALS
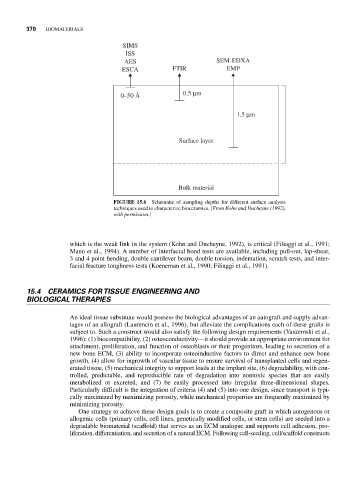
SIMS
ISS
AES SEM-EDXA
ESCA FTIR EMP
0–50 Å 0.5 μm
1.5 μm
Surface layer
Bulk material
FIGURE 15.6 Schematic of sampling depths for different surface analysis
techniques used to characterize bioceramics. [From Kohn and Ducheyne (1992),
with permission.]
which is the weak link in the system (Kohn and Ducheyne, 1992), is critical (Filiaggi et al., 1991;
Mann et al., 1994). A number of interfacial bond tests are available, including pull-out, lap-shear,
3 and 4 point bending, double cantilever beam, double torsion, indentation, scratch tests, and inter-
facial fracture toughness tests (Koeneman et al., 1990; Filiaggi et al., 1991).
15.4 CERAMICS FOR TISSUE ENGINEERING AND
BIOLOGICAL THERAPIES
An ideal tissue substitute would possess the biological advantages of an autograft and supply advan-
tages of an allograft (Laurencin et al., 1996), but alleviate the complications each of these grafts is
subject to. Such a construct would also satisfy the following design requirements (Yaszemski et al.,
1996): (1) biocompatibility, (2) osteoconductivity—it should provide an appropriate environment for
attachment, proliferation, and function of osteoblasts or their progenitors, leading to secretion of a
new bone ECM, (3) ability to incorporate osteoinductive factors to direct and enhance new bone
growth, (4) allow for ingrowth of vascular tissue to ensure survival of transplanted cells and regen-
erated tissue, (5) mechanical integrity to support loads at the implant site, (6) degradability, with con-
trolled, predictable, and reproducible rate of degradation into nontoxic species that are easily
metabolized or excreted, and (7) be easily processed into irregular three-dimensional shapes.
Particularly difficult is the integration of criteria (4) and (5) into one design, since transport is typi-
cally maximized by maximizing porosity, while mechanical properties are frequently maximized by
minimizing porosity.
One strategy to achieve these design goals is to create a composite graft in which autogenous or
allogenic cells (primary cells, cell lines, genetically modified cells, or stem cells) are seeded into a
degradable biomaterial (scaffold) that serves as an ECM analogue and supports cell adhesion, pro-
liferation, differentiation, and secretion of a natural ECM. Following cell-seeding, cell/scaffold constructs