Page 395 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 395
372 BIOMATERIALS
A bonelike apatite layer can be formed in vitro at STP conditions (Murphy et al., 2000a; Shin
et al., 2007; Abe et al., 1990; Li et al., 1992; Bunker et al., 1994; Campbell et al., 1996; Tanahashi
et al., 1995; Yamamoto et al., 1997; Wu et al., 1997; Wen et al., 1997), providing a way to control
the in vivo response to a biomaterial. The basis for synthesizing bonelike mineral in a biomimetic
fashion lies in the observation that in nature, organisms use macromolecules to control mineral nucle-
ation and growth (Weiner, 1986; Bunker et al., 1994). Macromolecules usually contain functional
groups that are negatively charged at the crystallization pH (Weiner, 1986), enabling them to chelate
ions present in the surrounding media which stimulate crystal nucleation (Bunker et al., 1994). The
key requirement is to chemically modify a substrate to induce heterogeneous nucleation of mineral
from a solution (Bunker et al., 1994). Biomimetic processes are guided by the pH and ionic con-
centration of the microenvironment, and conditions conducive to heterogeneous nucleation will
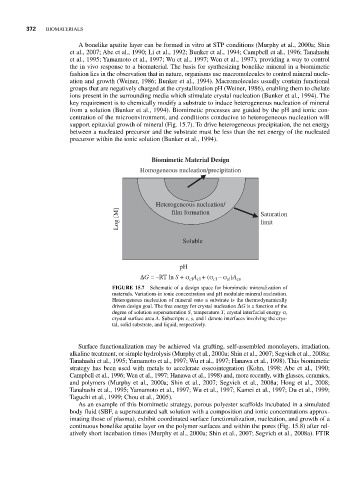
support epitaxial growth of mineral (Fig. 15.7). To drive heterogeneous precipitation, the net energy
between a nucleated precursor and the substrate must be less than the net energy of the nucleated
precursor within the ionic solution (Bunker et al., 1994).
Biomimetic Material Design
Homogeneous nucleation/precipitation
Heterogeneous nucleation/
Log [M] film formation Saturation
limit
Soluble
pH
ΔG = –RT ln S + σ A + (σ – σ )A cs
cl
cl cl
sl
FIGURE 15.7 Schematic of a design space for biomimetic mineralization of
materials. Variations in ionic concentration and pH modulate mineral nucleation.
Heterogenous nucleation of mineral onto a substrate is the thermodynamically
driven design goal. The free energy for crystal nucleation ΔG is a function of the
degree of solution supersaturation S, temperature T, crystal interfacial energy σ,
crystal surface area A. Subscripts c, s, and l denote interfaces involving the crys-
tal, solid substrate, and liquid, respectively.
Surface functionalization may be achieved via grafting, self-assembled monolayers, irradiation,
alkaline treatment, or simple hydrolysis (Murphy et al., 2000a; Shin et al., 2007; Segvich et al., 2008a;
Tanahashi et al., 1995; Yamamoto et al., 1997; Wu et al., 1997; Hanawa et al., 1998). This biomimetic
strategy has been used with metals to accelerate osseointegration (Kohn, 1998; Abe et al., 1990;
Campbell et al., 1996; Wen et al., 1997; Hanawa et al., 1998) and, more recently, with glasses, ceramics,
and polymers (Murphy et al., 2000a; Shin et al., 2007; Segvich et al., 2008a; Hong et al., 2008;
Tanahashi et al., 1995; Yamamoto et al., 1997; Wu et al., 1997; Kamei et al., 1997; Du et al., 1999;
Taguchi et al., 1999; Chou et al., 2005).
As an example of this biomimetic strategy, porous polyester scaffolds incubated in a simulated
body fluid (SBF, a supersaturated salt solution with a composition and ionic concentrations approx-
imating those of plasma), exhibit coordinated surface functionalization, nucleation, and growth of a
continuous bonelike apatite layer on the polymer surfaces and within the pores (Fig. 15.8) after rel-
atively short incubation times (Murphy et al., 2000a; Shin et al., 2007; Segvich et al., 2008a). FTIR