Page 396 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 396
BIOCERAMICS 373
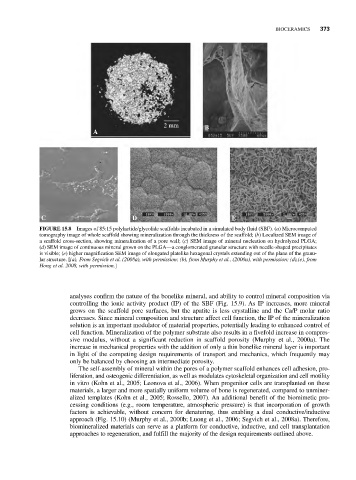
2 mm B
A
C D E
FIGURE 15.8 Images of 85:15 polylactide/glycolide scaffolds incubated in a simulated body fluid (SBF). (a) Microcomputed
tomography image of whole scaffold showing mineralization through the thickness of the scaffold; (b) Localized SEM image of
a scaffold cross-section, showing mineralization of a pore wall; (c) SEM image of mineral nucleation on hydrolyzed PLGA;
(d) SEM image of continuous mineral grown on the PLGA—a conglomerated granular structure with needle-shaped precipitates
is visible; (e) higher magnification SEM image of elongated platelike hexagonal crystals extending out of the plane of the granu-
lar structure. [(a), From Segvich et al. (2008a), with permission; (b), from Murphy et al., (2000a), with permission; (d),(e), from
Hong et al. 2008, with permission.]
analyses confirm the nature of the bonelike mineral, and ability to control mineral composition via
controlling the ionic activity product (IP) of the SBF (Fig. 15.9). As IP increases, more mineral
grows on the scaffold pore surfaces, but the apatite is less crystalline and the Ca/P molar ratio
decreases. Since mineral composition and structure affect cell function, the IP of the mineralization
solution is an important modulator of material properties, potentially leading to enhanced control of
cell function. Mineralization of the polymer substrate also results in a fivefold increase in compres-
sive modulus, without a significant reduction in scaffold porosity (Murphy et al., 2000a). The
increase in mechanical properties with the addition of only a thin bonelike mineral layer is important
in light of the competing design requirements of transport and mechanics, which frequently may
only be balanced by choosing an intermediate porosity.
The self-assembly of mineral within the pores of a polymer scaffold enhances cell adhesion, pro-
liferation, and osteogenic differentiation, as well as modulates cytoskeletal organization and cell motility
in vitro (Kohn et al., 2005; Leonova et al., 2006). When progenitor cells are transplanted on these
materials, a larger and more spatially uniform volume of bone is regenerated, compared to unminer-
alized templates (Kohn et al., 2005; Rossello, 2007). An additional benefit of the biomimetic pro-
cessing conditions (e.g., room temperature, atmospheric pressure) is that incorporation of growth
factors is achievable, without concern for denaturing, thus enabling a dual conductive/inductive
approach (Fig. 15.10) (Murphy et al., 2000b; Luong et al., 2006; Segvich et al., 2008a). Therefore,
biomineralized materials can serve as a platform for conductive, inductive, and cell transplantation
approaches to regeneration, and fulfill the majority of the design requirements outlined above.