Page 252 - Biomimetics : Biologically Inspired Technologies
P. 252
Bar-Cohen : Biomimetics: Biologically Inspired Technologies DK3163_c008 Final Proof page 238 21.9.2005 3:08am
238 Biomimetics: Biologically Inspired Technologies
hydrophilic head and six valines as the hydrophobic tail. Leucine and isoleucines are also used as
tails. Positively charged lysine and histidine and negatively charged aspartic acid and glutamic acids
have also been used as heads. (Vauthey et al., 2002; Santoso et al., 2002; von Maltzahn et al., 2003).
These peptides undergo self-assembly in water to form nanotubes and nanovesicles having an
average diameter of 30 to 50 nm (Vauthey et al., 2002; Santoso et al., 2002; von Maltzahn et al.,
2003). The tails consisting of alanines and valines produce more homogeneous and stable structures
than those of glycines, isoleucine, and leucine. This property may be due to their hydrophobic and
hydrophilic ratios. These monomer surfactant peptides were used for molecular modeling. The
negatively charged aspartic acid is modeled as red and positively lysine is blue with the green as the
hydrophobic tails.
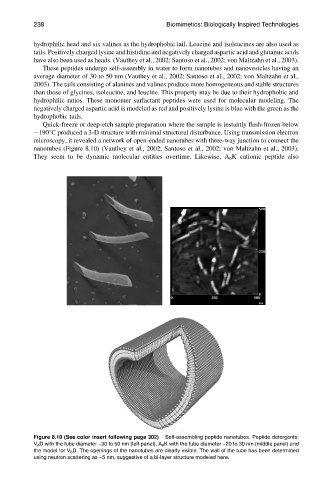
Quick-freeze or deep-etch sample preparation where the sample is instantly flash-frozen below
1908C produced a 3-D structure with minimal structural disturbance. Using transmission electron
microscopy, it revealed a network of open-ended nanotubes with three-way junction to connect the
nanotubes (Figure 8.10) (Vauthey et al., 2002; Santoso et al., 2002; von Maltzahn et al., 2003).
They seem to be dynamic molecular entities overtime. Likewise, A 6 K cationic peptide also
Figure 8.10 (See color insert following page 302) Self-assembling peptide nanotubes. Peptide detergents:
V 6 D with the tube diameter ~30 to 50 nm (left panel), A 6 K with the tube diameter ~20 to 30 nm (middle panel) and
the model for V 6 D. The openings of the nanotubes are clearly visible. The wall of the tube has been determined
using neutron scattering as ~5 nm, suggestive of a bi-layer structure modeled here.