Page 59 - Biomimetics : Biologically Inspired Technologies
P. 59
Bar-Cohen : Biomimetics: Biologically Inspired Technologies DK3163_c002 Final Proof page 45 21.9.2005 11:39am
Biomimetics of Muscle Design 45
major determinants of muscle function. Other important structures within muscle cells are the
mitochondria that are responsible for the aerobe energy metabolism and the sarcoplasmic reticulum
(SR), which plays a crucial role in the activation and relaxation kinetics of muscle. It is known that
changes in the volume fraction of mitochondria, SR, and myofibrillar proteins can be utilized to
modify muscle function (Conley and Lindstedt, 2002). For example, in high-frequency muscles
involved in sound production, the SR fraction is enlarged at the expense of the myofibrillar protein
fraction to attain superfast muscle contraction (Conley and Lindstedt, 2002). This kind of special-
ization will not be dealt with in this chapter. Instead the remainder of this chapter will focus on the
design and organization of the sarcomeres, and it will be discussed how the natural design might
provide inspiration for artificial muscles.
2.3.1 The Sarcomere
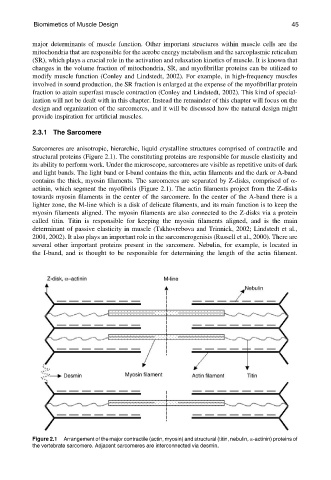
Sarcomeres are anisotropic, hierarchic, liquid crystalline structures comprised of contractile and
structural proteins (Figure 2.1). The constituting proteins are responsible for muscle elasticity and
its ability to perform work. Under the microscope, sarcomeres are visible as repetitive units of dark
and light bands. The light band or I-band contains the thin, actin filaments and the dark or A-band
contains the thick, myosin filaments. The sarcomeres are separated by Z-disks, comprised of a-
actinin, which segment the myofibrils (Figure 2.1). The actin filaments project from the Z-disks
towards myosin filaments in the center of the sarcomere. In the center of the A-band there is a
lighter zone, the M-line which is a disk of delicate filaments, and its main function is to keep the
myosin filaments aligned. The myosin filaments are also connected to the Z-disks via a protein
called titin. Titin is responsible for keeping the myosin filaments aligned, and is the main
determinant of passive elasticity in muscle (Tskhovrebova and Trinnick, 2002; Lindstedt et al.,
2001, 2002). It also plays an important role in the sarcomerogenisis (Russell et al., 2000). There are
several other important proteins present in the sarcomere. Nebulin, for example, is located in
the I-band, and is thought to be responsible for determining the length of the actin filament.
Figure 2.1 Arrangement of the major contractile (actin, myosin) and structural (titin, nebulin, a-actinin) proteins of
the vertebrate sarcomere. Adjacent sarcomeres are interconnected via desmin.