Page 290 - Biosystems Engineering
P. 290
Biodiesel and Ethanol in Engines 267
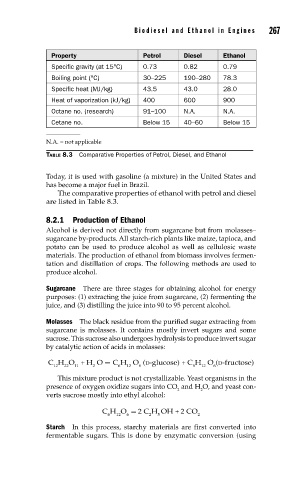
Property Petrol Diesel Ethanol
Specific gravity (at 15°C) 0.73 0.82 0.79
Boiling point (°C) 30–225 190–280 78.3
Specific heat (MJ/kg) 43.5 43.0 28.0
Heat of vaporization (kJ/kg) 400 600 900
Octane no. (research) 91–100 N.A. N.A.
Cetane no. Below 15 40–60 Below 15
N.A. = not applicable
TABLE 8.3 Comparative Properties of Petrol, Diesel, and Ethanol
Today, it is used with gasoline (a mixture) in the United States and
has become a major fuel in Brazil.
The comparative properties of ethanol with petrol and diesel
are listed in Table 8.3.
8.2.1 Production of Ethanol
Alcohol is derived not directly from sugarcane but from molasses–
sugarcane by-products. All starch-rich plants like maize, tapioca, and
potato can be used to produce alcohol as well as cellulosic waste
materials. The production of ethanol from biomass involves fermen-
tation and distillation of crops. The following methods are used to
produce alcohol.
Sugarcane There are three stages for obtaining alcohol for energy
purposes: (1) extracting the juice from sugarcane, (2) fermenting the
juice, and (3) distilling the juice into 90 to 95 percent alcohol.
Molasses The black residue from the purified sugar extracting from
sugarcane is molasses. It contains mostly invert sugars and some
sucrose. This sucrose also undergoes hydrolysis to produce invert sugar
by catalytic action of acids in molasses:
C H O + H O = C H O (D-glucose) + C H O (D-fructose)
12 22 11 2 6 12 6 6 12 6
This mixture product is not crystallizable. Yeast organisms in the
presence of oxygen oxidize sugars into CO and H O, and yeast con-
2 2
verts sucrose mostly into ethyl alcohol:
C H O = 2 C H OH + 2 CO
6 12 6 2 5 2
Starch In this process, starchy materials are first converted into
fermentable sugars. This is done by enzymatic conversion (using