Page 403 - Biosystems Engineering
P. 403
380 Cha pte r T h i r tee n
3. Reversion—a type of flavor and odor degradation usually
associated with the oxidation of certain highly unsaturated
vegetable oils (e.g., soybean oil) and fish oils. This flavor deg-
radation is attributed to oxidation of linolenic acid, and other
omega-3 fatty acids.
4. Polymerization—a term usually used to describe the cross-
linking of unsaturated fats between two carbon atoms. Poly-
mers are also formed with oxygen between two fatty acid
chains at an unsaturated site with both types of polymers
containing cyclic structures.
13.5.1 Mechanism of Antioxidants
Generally, the oxidation of fats or oils involves a free-radical mecha-
nism. This process can be induced catalytically by light, temperature,
enzymes, metals, metalloproteins, and microorganisms, with the
reaction involving free radicals or active oxygen species. 20
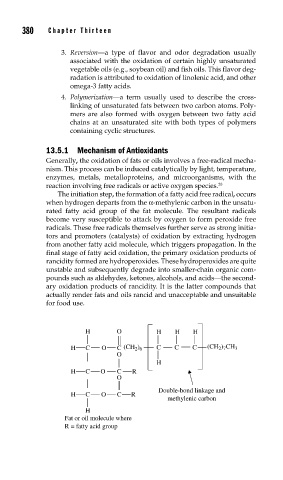
The initiation step, the formation of a fatty acid free radical, occurs
when hydrogen departs from the α-methylenic carbon in the unsatu-
rated fatty acid group of the fat molecule. The resultant radicals
become very susceptible to attack by oxygen to form peroxide free
radicals. These free radicals themselves further serve as strong initia-
tors and promoters (catalysts) of oxidation by extracting hydrogen
from another fatty acid molecule, which triggers propagation. In the
final stage of fatty acid oxidation, the primary oxidation products of
rancidity formed are hydroperoxides. These hydroperoxides are quite
unstable and subsequently degrade into smaller-chain organic com-
pounds such as aldehydes, ketones, alcohols, and acids—the second-
ary oxidation products of rancidity. It is the latter compounds that
actually render fats and oils rancid and unacceptable and unsuitable
for food use.
H O H H H
H C O C (CH ) C C C (CH ) CH 3
2 6
2 7
O
H
H C O C R
O
Double-bond linkage and
H C O C R
methylenic carbon
H
Fat or oil molecule where
R = fatty acid group