Page 188 - Carbon Nanotubes
P. 188
et
180 U. ZIMMERMAN ai.
preted to signal the onset of metal-metal bonding. An
exception to the electronically determined cluster sta-
bility is C60Li12, which was observed to be particularly
stable independent of the cluster charge. Supported by
MNDO calculations, we found that the geometrical ar-
rangement of atoms in this cluster, one above each
pentagon of the fullerene, was most important for the
stability. At higher alkali metal coverage of the ful-
lerene, an electronic shell structure similar to pure
metal clusters is observed in the ionization threshold
of the clusters.
Acknowledgements-We would like to thank H. Schaber for
his outstanding technical assistance, U. Naher for many stim-
ulating discussions, and A. Mittelbach for providing the C,,
used in the experiments.
,
IS’ I,, , , , , , , , , REFERENCES
0.0 0.2 0.4 0.6 0.8
RCJR0.t 1. A. E Hebard, M. J. Rosseinsky, R. C. Haddon, D W.
Murphy, S. H. Glarum, T. T. Palstra, A. P. Ramirez,
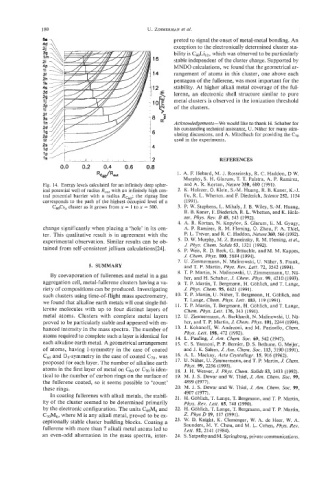
Fig. 14. Energy levels calculated for an infinitely deep spher- and A. R. Kortan, Nature 350, 600 (1991).
ical potential well of radius R,,, with an infinitely high cen- 2. K. Holczer, 0. Klein, S.-M. Huang, R. E. Kaner, K.4.
tral potential barrier with a radius Rc6,; the zigzag line Fu, R. L. Whetten, and E Diederich, Science 252, 1154
corresponds to the path of the highest occupied level of a (1991).
C,Cs, cluster as it grows from x = 1 to x = 500. 3. P. W. Stephens, L. Mihaly, J. B. Wiley, S.-M. Huang,
R. B. Kaner, E Diederich, R. L. Whetten, and K. Holc-
zer, Phys. Rev. B 45, 543 (1992).
4. A. R. Kortan, N. Kopylov, S. Glarum, E. M. Gyogy,
change significantly when placing a ‘hole’ in its cen- A. P. Ramirez, R. M. Fleming, 0. Zhou, E A. Thiel,
ter. This qualitative result is in agreement with the P. L. Trevor, and R. C. Haddon, Nature 360,566 (1992).
experimental observation. Similar results can be ob- 5. D. W. Murphy, M. Z. Rosseinsky, R. M. Fleming, eta/.,
J. Phys. Chem. Solids 53, 1321 (1992).
tained from self-consistent jellium calculations[%]. 6. P. Weis, R. D. Beck, G. Brauchle, and M. M. Kappes,
J. Chem. Phys. 100, 5684 (1994).
7. U. Zimmermann, N. Malinowski, U. Naher, S. Frank,
5. SUMMARY and T. P. Martin, Phys. Rev. Lett. 72, 3542 (1994).
8. T. P. Martin, N. Malinowski, U. Zimmermann, U. Na-
By coevaporation of fullerenes and metal in a gas her, and H. Schaber, J. Chem. Phw. 99. 4210 (1993).
aggregation cell, metal-fullerene clusters having a va- 9. T. P. Martin, T. Bergmann, H. Gohlich, and T. Lange,
riety of compositions can be produced. Investigating J. Phys. Chem. 95, 6421 (1991).
such clusters using time-of-flight mass spectrometry, 10. T. P. Martin, U. Naher, T. Bergmann, H. Gohlich, and
T. Lange, Chem. Phys. Lett. 183, 119 (1991).
we found that alkaline earth metals will coat single ful- 11. T. P. Martin, T. Bergmann, H. Gohlich, and T. Lange,
lerene molecules with up to four distinct layers of Chem. Phys. Lett. 176, 343 (1991).
metal atoms. Clusters with complete metal layers 12. U. Zimmermann, A. Burkhardt, N. Malinowski, U. Na-
proved to be particularly stable and appeared with en- her, and T. P. Martin, J. Chem. Phys. 101, 2244 (1994).
hanced intensity in the mass spectra. The number of 13. J. Kohanoff, W. Andreoni, and M. Parinello, Chem.
Phys. Lett. 198, 472 (1992).
atoms required to complete such a layer is identical for 14. L. Pauling, J. Am. Chem. SOC. 69, 542 (1947).
each alkaline earth metal. A geometrical arrangement 15. C. S. Yannoni, P. P. Bernier, D. S. Bethune, G. Meijer,
of atoms, having I-symmetry in the case of coated and J. K. Salem, J. Am. Chem. Soc. 113, 3190 (1991).
c60 and D,-symmetry in the case of coated C,,, was 16. A. L. Mackay, Acta Crystallogr. 15, 916 (1962).
proposed for each layer. The number of alkaline earth 17. U. Naher, U. Zimmermann, and T. P. Martin, J. Chem.
Phys. 99, 2256 (1993).
atoms in the first layer of metal on c60 or C70 is iden- 18. J. H. Weaver, J. Phys. Chem. Solids 53, 1433 (1992).
tical to the number of carbon rings on the surface of 19. M. J. S. Dewar and W. Thiel, J. Am. Chem. SOC. 99,
the fullerene coated, so it seems possible to ‘count’ 4899 (1977).
these rings. 20. M. J. S. Dewar and W. Thiel, J. Am. Ckem. SOC. 99,
4907 (1977).
In coating fullerenes with alkali metals, the stabil- 21. H. Gohlich, T. Lange, T. Bergmann, and T. P. Martin,
ity of the cluster seemed to be determined primarily Phys. Rev. Lett. 65, 748 (1990).
by the electronic configuration. The units C&i6 and 22. H. Gohlich, T. Lange, T. Bergmann, and T. P. Martin,
C7oM6, where M is any alkali metal, proved to be ex- Z. Phys D 19, 117 (1991).
ceptionally stable cluster building blocks. Coating a 23. W. D. Knight, K. Clemenger, W. A. de Heer, W. A.
Saunders, M. Y. Chou, and M. L. Cohen, Phys. Rev.
fullerene with more than 7 alkali metal atoms led to Lett. 52, 2141 (1984).
an even-odd alternation in the mass spectra, inter- 24. S. Satpathy and M. Springborg, private communications.