Page 185 - Carbon Nanotubes
P. 185
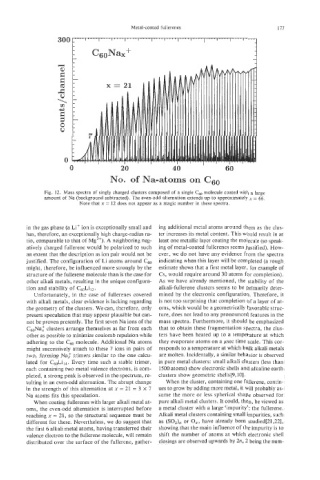
0 20 40 60
No. of Na-atoms on CG0
Fig. 12. Mass spectra of singly charged clusters composed of a single C, molecule coated with a large
amount of Na (background subtracted). The even-odd alternation extends up to approximately x = 66.
Note that x = 12 does not appear as a magic number in these spectra.
in the gas phase (a Li' ion is exceptionally small and ing additional metal atoms around them as the clus-
has, therefore, an exceptionally high charge-radius ra- ter increases its metal content. This would result in at
tio, comparable to that of Mg2+). A neighboring neg- least one metallic layer coating the molecule (so speak-
atively charged fuIlerene would be polarized to such ing of metal-coated fullerenes seems justified). How-
an extent that the description as ion pair would not be ever, we do not have any evidence from the spectra
justified. The configuration of Li atoms around Cb0 indicating when this layer will be completed (a rough
might, therefore, be influenced more strongly by the estimate shows that a first metal layer, for example of
structure of the fullerene molecule than is the case for Cs, would require around 30 atoms for completion).
other alkali metals, resulting in the unique configura- As we have already mentioned, the stability of the
tion and stability of C6,,Li12. alkali-fullerene clusters seems to be primarily deter-
TJnfortunately, in the case of fullerenes covered mined by the electronic configuration. Therefore, it
with alkali metals, clear evidence is lacking regarding is not too surprising that completion of a Payer of at-
the geometry of the clusters. We can, therefore, only oms, which would be a geometrically favorable struc-
present speculation that may appear plausible but can- ture, does not lead to any pronounced features in the
not be proven presently. The first seven Na ions of the mass spectra. Furthermore, it should be emphasized
C,Na: clusters arrange themselves as far from each that to obtain these fragmentation spectra, the clus-
other as possible to minimize coulomb repulsion while ters have been heated up to a temperature at which
adhering to the C, molecule. Additional Na atoms they evaporate atoms on a psec time scale. This cor-
might successively attach to these 7 ions in pairs of responds to a temperature at which bulk alkali metals
two, forming Na: trimers similar to the one calcu- are molten. Incidentally, a similar behavior is observed
lated for Cs0Lil4. Every time such a stable trimer, in pure metal clusters: small alkali clusters (less than
each containing two metal valence electrons, is com- 1500 atoms) show electronic shells and alkaline earth
pleted, a strong peak is observed in the spectrum, re- clusters show geometric shells[9,10].
sulting in an even-odd alternation. The abrupt change When the cluster, containing one fullerene, contin-
in the strength of this alternation at x = 21 = 3 X 7 ues to grow by adding more metal, it will probably as-
Na atoms fits this speculation. sume the more or less spherical shape observed for
When coating fuIlerenes with larger alkali metal at- pure alkali metal clusters. It could, then, be viewed as
oms, the even-odd alternation is interrupted before a metal cluster with a large 'impurity': the fullerene.
reaching x = 21, so the structural sequence must be Alkali metal clusters containing small impurities, such
different for these. Nevertheless, we do suggest that as (SO,), or On, have already been studied[21,22],
the first 6 alkali metal atoms, having transferred their showing that the main influence of the impurity is to
valence electron to the fullerene molecule, will remain shift the number of atoms at which electronic shell
distributed over the surface of the fullerene, gather- closings are observed upwards by 2n, 2 being the num-