Page 183 - Carbon Nanotubes
P. 183
Metal-coated fullerenes 175
300 3
2000 1
LixC,+,
Y
v)
FI
2
0
0 2000 4000 ' 6000 ' 8000
mass [amu] 300
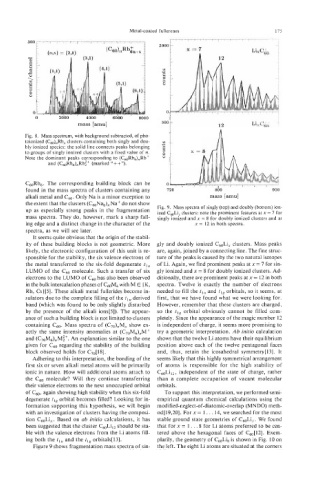
Fig. 8. Mass spectrum, with background subtracted, of pho-
toionized (C,),Rb, clusters containing both singly and dou- Y
v)
bly ionized species: the solid line connects peaks belonging 0
to groups of singly ionized clusters with a fixed value of n. 1
8
Note the dominant peaks corresponding to (c,&b6),Rb+
and (C60Rb6),Rb$+ (marked 'I++").
C6,Rb6. The corresponding building block can be 0
found in the mass spectra of clusters containing any 720 so0 900
alkali metal and Cm. Only Na is a minor exception to mass [amu]
the extent that the clusters (c60Na6),,Naf do not show
up as especially strong peaks in the fragmentation Fig. 9. Mass spectra of singly (top) and doubly (bottom) ion-
clusters: note the prominent features at x = 7 for
ized C,Li,
mass spectra. They do, however, mark a sharp fall- singly ionized and x = 8 for doubly ionized clusters and at
ing edge and a distinct change in the character of the x = 12 in both spectra.
spectra, as we will see later.
It seems quite obvious that the origin of the stabil-
ity of these building blocks is not geometric. More gly and doubly ionized CmLiw clusters. Mass peaks
likely, the electronic configuration of this unit is re- are, again, joined by a connecting line. The fine struc-
sponsible for the stability, the six valence electrons of ture of the peaks is caused by the two natural isotopes
the metal transferred to the six-fold degenerate t, , of Li. Again, we find prominent peaks at x = 7 for sin-
LUMO of the c60 molecule. Such a transfer of six gly ionized and x = 8 for doubly ionized clusters. Ad-
electrons to the LUMO of Cm has also been observed ditionally, there are prominent peaks at x = 12 in both
in the bulk intercalation phases of C60M6 with M E (K, spectra. Twelve is exactly the number of electrons
Rb, Cs)[5]. These alkali metal fullerides become in- needed to fill the t,, and t,, orbitals, so it seems, at
sulators due to the complete filling of the t,, derived first, that we have found what we were looking for.
band (which was found to be only slightly disturbed However, remember that these clusters are charged,
by the presence of the alkali ions[5]). The appear- so the tl, orbital obviously cannot be filled com-
ance of such a building block is not limited to clusters pletely. Since the appearance of the magic number 12
containing c60. Mass spectra of (C70)nMx show ex- is independent of charge, it seems more promising to
actly the same intensity anomalies at (C70M6)nM+ try a geometric interpretation. Ab initio calculation
and (C70M6)nM:+. An explanation similar to the one shows that the twelve Li atoms have their equilibrium
given for c60 regarding the stability of the building position above each of the twelve pentagonal faces
block observed holds for C,,[18]. and, thus, retain the icosahedral symmetry[l3]. It
Adhering to this interpretation, the bonding of the seems likely that this highly symmetrical arrangement
first six or seven alkali metal atoms will be primarily of atoms is responsible for the high stability of
ionic in nature. How will additional atoms attach to C60LilL, independent of the state of charge, rather
the c60 molecule? Will they continue transferring than a complete occupation of vacant molecular
their valence electrons to the next unoccupied orbital orbitals.
of Cmr again showing high stability when this six-fold To support this interpretation, we performed semi-
degenerate tl, orbital becomes filled? Looking for in- empirical quantum chemical calculations using the
formation supporting this hypothesis, we will begin modified-neglect-of-diatomic-overlap (MNDO) meth-
with an investigation of clusters having the composi- od[19,20]. For x = 1 . . . 14, we searched for the most
tion CbOLix. Based on ab initio calculations, it has stable ground state geometries of C,,Li,. We found
been suggested that the cluster C60Li12 should be sta- that for x = 1 . . .8 for Li atoms preferred to be cen-
ble with the valence electrons from the Li atoms fill- tered above the hexagonal faces of c60[12]. Exem-
ing both the t,, and the t,, orbitals[l3]. plarily, the geometry of C60Li8 is shown in Fig. 10 on
Figure 9 shows fragmentation mass spectra of sin- the left. The eight Li atoms are situated at the corners