Page 178 - Carbon Nanotubes
P. 178
170 U. ZIMMERMAN al.
et
a two-stage reflector and a mass resolution of better
than 20,000.
REF The size distribution of the clusters produced in the
7 cluster source is quite smooth, containing no informa-
tion about the clusters except their composition. To
obtain information about, for example, the relative
stability of clusters, it is often useful to heat the clus-
ters. Hot clusters will evaporate atoms and molecules,
preferably until a more stable cluster composition is
reached that resists further evaporation. This causes
an increase in abundance of the particularly stable spe-
cies (Le., enhancing the corresponding peak in the
C LERATION REOlON
mass spectrum, then commonly termed 'fragmentation
" spectrum'). Using sufficiently high laser fluences
CLUSTER PUMPING TOF MASS (=50 pJ/mm2), the clusters can be heated and ion-
CONDENSATION STAGE SPECIXOMETER
CELL ized simultaneously with one laser pulse.
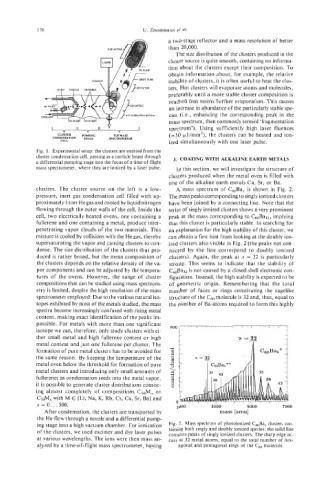
Fig. 1. Experimental setup: the clusters are emitted from the
cluster condensation cell, passing as a particle beam through
a differential pumping stage into the focus of a time-of-flight 3. COATING WITH ALKALINE EARTH METALS
mass spectrometer, where they are ionized by a laser pulse. In this section, we will investigate the structure of
clusters produced when the metal oven is filled with
one of the alkaline earth metals Ca, Sr, or Ba.
clusters. The cluster source on the left is a low- A mass spectrum of C60Bax is shown in Fig. 2.
pressure, inert gas condensation cell filled with ap- The mass peaks corresponding to singly ionized clusters
proximately 1 torr He gas and cooled by liquid nitrogen have been joined by a connecting line. Note that the
flowing through the outer walls of the cell. Inside the series of singly ionized clusters shows a very prominent
cell, two electrically heated ovens, one containing a peak at the mass corresponding to C60Ba32, implying
fullerene and one containing a metal, produce inter- that this cluster is particularly stable. In searching for
penetrating vapor clouds of the two materials. This an explanation for the high stability of this cluster, we
mixture is cooled by collisions with the He gas, thereby can obtain a first hint from looking at the doubly ion-
supersaturating the vapor and causing clusters to con- ized clusters also visible in Fig. 2 (the peaks not con-
dense. The size distribution of the clusters thus pro- nected by the line correspond to doubly ionized
duced is rather broad, but the mean composition of clusters). Again, the peak at x = 32 is particularly
the clusters depends on the relative density of the va- strong. This seems to indicate that the stability of
por components and can be adjusted by the tempera- CmBa,, is not caused by a closed-shell electronic con-
tures of the ovens. However, the range of cluster figuration. Instead, the high stability is expected to be
compositions that can be studied using mass spectrom- of geometric origin. Remembering that the total
etry is limited, despite the high resolution of the mass number of faces or rings constituting the cagelike
spectrometer employed: Due to the various natural iso- structure of the c60 molecule is 32 and, thus, equal to
topes exhibited by most of the metals studied, the mass the number of Ba-atoms required to form this highly
spectra become increasingly confused with rising metal
content, making exact identification of the peaks im-
possible. For metals with more than one significant
isotope we can, therefore, only study clusters with ei- 800
ther small metal and high fullerene content or high
metal content and just one fullerene per cluster. The -
formation of pure metal clusters has to be avoided for :
the same reason. By keeping the temperature of the 9
metal oven below the threshold for formation of pure -3
metal clusters and introducing only small amounts of -2
c
fullerenes as condensation seeds into the metal vapor, Y
1
it is possible to generate cluster distributions consist- 8
ing almost completely of compositions C60Mx or
C70Mx with M E (Li, Na, K, Rb, Cs, Ca, Sr, Ba) and
x=o ... 500. 0 3000 5000 7000
1000
After condensation, the clusters are transported by mass [amu]
the He-flow through a nozzle and a differential pump-
ing stage into a high vacuum chamber. For ionization Fig. 2. Mass spectrum of photoionized C,Ba, clusters con-
of the clusters, we used excimer and dye laser pulses taining both singly and doubly ionized species: the solid line
connects peaks of singly ionized clusters. The sharp edge oc-
at various wavelengths. The ions were then mass an- curs at 32 metal atoms, equal to the total number of hex-
alyzed by a time-of-flight mass spectrometer, having agonal and pentagonal rings of the C60 molecule.