Page 179 - Carbon Nanotubes
P. 179
Metal-coated fullerenes 171
I
stable cluster, the arrangement of metal atoms in this 300 1
cluster becomes obvious. By placing one Ba atom onto
x = 32
each of the 12 pentagons and 20 hexagons of the c60
molecule, a structure with full icosahedral symmetry
(point group I,,) is obtained that can be visualized as 104 -
an almost close-packed layer of 32 Ba-atoms coating
the c60 molecule. It seems reasonable that this struc-
ture exhibits an unusually high stability, somewhat
similar to the geometric shells observed in pure alka-
line earth clusters[lO,l I]. Any additional metal atoms
situated on this first metal layer are likely to be only
weakly bound to the layer underneath and, thus, evap-
orate easily, causing the mass peaks of C60Bax with x
greater than 32 to disappear almost completely. The
small peaks at x = 35,38, and 43 might signal the com-
pl’etion of small stable metal islands on the first metal
layer. We can, however, presently only speculate on
the nature of these minor structures.
For a rough estimate of the packing density of this
first metal layer, assume the atoms to be hard spheres
having the covalent radii of the respective atoms (0.77 A
for C; 1.98 A for Ba[14]). Placing the carbon spheres 0
at the appropriate sites of the Cm structure with bond 50 X 100 130
lengths 1.40 -4 and 1.45 A[ 151 and letting the Ba spheres
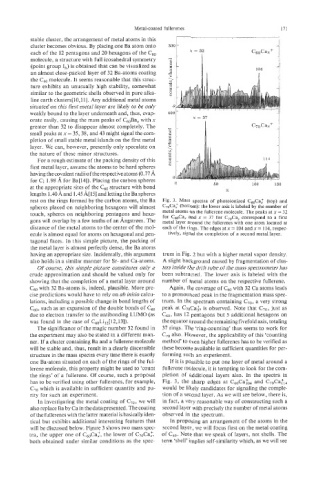
rest on the rings formed by the carbon atoms, the Ba Fig. 3. Mass spectra of photoionized C,,Ca; (top) and
spheres placed on neighboring hexagons will almost C7&a: (bottom): the lower axis is labeled by the number of
touch, spheres on neighboring pentagons and hexa- metal atoms on the fullerene molecule. The peaks at x = 32
correspond to a first
for CmCa, and x = 37 for C&a,
gons will overlap by a few tenths of an hgstr~m. The metal layer around the fullerenes with one atom located at
distance of the metal atoms to the center of the mol- each of the rings. The edges at x = 104 and x = 114, respec-
ecule is almost equal for atoms on hexagonal and pen- tively, signal the completion of a second metal layer.
tagonal faces. In this simple picture, the packing of
the metal layer is almost perfectly dense, the Ba atoms
having an appropriate size. Incidentally, this argument trum in Fig. 2 but with a higher metal vapor density.
also holds in a similar manner for Sr- and Ca-atoms. A slight background caused by fragmentation of clus-
Of course, this simple picture constitutes only a ters inside the drift tube of the mass spectrometer has
crude approximation and should be valued only for been subtracted. The lower axis is labeled with the
showing that the completion of a metal layer around number of metal atoms on the respective fullerene.
C60 with 32 Ba-atoms is, indeed, plausible. More pre- Again, the coverage of C6,, with 32 Ca atoms leads
cise predictions would have to rely on ab initio calcu- to a pronounced peak in the fragmentation mass spec-
lations, including a possible change in bond lengths of trum. In the spectrum containing C70, a very strong
C60, such as an expansion of the double bonds of C,jo peak at C70Ca:7 is observed. Note that C70, just as
due to electron transfer to the antibonding LUMO (as C60, has 12 pentagons but 5 additional hexagons on
was found in the case of C60Li,2[12,13]). the equator around the remaining fivefold axis, totaling
The significance of the magic number 32 found in 37 rings. The ‘ring-counting’ thus seems to work for
the experiment may also be stated in a different man- C70 also. However, the applicability of this ‘counting
ner. If a cluster containing Ba and a fullerene molecule method’ to even higher fullerenes has to be verified as
will be stable and, thus, result in a clearly discernible these become available in sufficient quantities for per-
structure in the mass spectra every time there is exactly forming such an experiment.
one Ba-atom situated on each of the rings of the ful- If it is possible to put one layer of metal around a
lerene molecule, this property might be used to ‘count fullerene molecule, it is tempting to look for the com-
the rings’ of a fullerene. Of course, such a proposal pletion of additional layers also. In the spectra in
has to be verified using other fullerenes, for example, Fig. 3, the sharp edges at C60Ca:04 and C70Ca~,,
C70 which is available in sufficient quantity and pu- would be likely candidates for signaling the comple-
rity for such an experiment. tion of a second layer. As we will see below, there is,
In investigating the metal coating of C70, we will in fact, a very reasonable way of constructing such a
also replace Ba by Cain the data presented. The coating second layer with precisely the number of metal atoms
of the fullerenes with the latter material is basically iden- observed in the spectrum.
tical but exhibits additional interesting features that In proposing an arrangement of the atoms in the
will be discussed below. Figure 3 shows two mass spec- second layer, we will focus first on the metal coating
tra, the upper one of C,,Ca:, the lower of C70Ca;, of C60. Note that we speak of layers, not shells. The
both obtained under similar conditions as the spec- term ‘shell’ implies self-similarity which, as we will see