Page 181 - Carbon Nanotubes
P. 181
Metal-coated fullerenes 173
dex K. Define K as the number of atoms along the
edge of a triangular face without including the atoms
on the vertices above the C60-pentagons. The first
layer then has K = 1, the second K = 2. The number
of atoms in the Kth layer can easily be calculated to
10K2 + 10K + 12. (1)
C60M32 K=3
C60M104
C60M236 The total number of atoms N(K) in a cluster com-
posed of K complete layers around c60 becomes
N(K) = i(10K3 + 30K2 + 56K). (2)
Note that the coefficient of the leading order in K, de-
termining the shell spacing on an N1'3 scale, is equal to
that of an icosahedral cluster of the Mackay type[l7].
Inserting K = 1 . . .4 into eqn (2), we find N( 1) = 32,
N(2) = 104, N(3) = 236, N(4) = 448, N(5) = 760, etc.
Did we predict the number of atoms required to
complete additional layers around the metal-coated
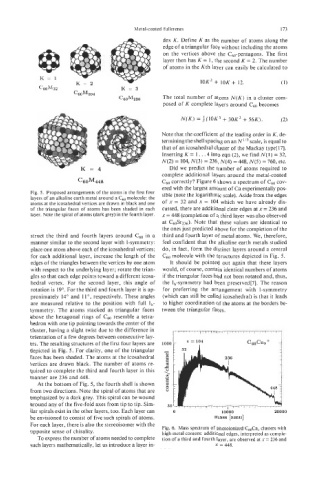
C60M448 c60 correctly? Figure 6 shows a spectrum of c60 cov-
ered with the largest amount of Ca experimentally pos-
Fig. 5. Proposed arrangements of the atoms in the first four sible (note the logarithmic scale). Aside from the edges
layers of an alkaline earth metal around a Cm molecule: the
atoms at the icosahedral vertices are drawn in black and one of x = 32 and x = 104 which we have already dis-
of the triangular faces of atoms has been shaded in each cussed, there are additional clear edges at x = 236 and
layer. Note the spiral of atoms (dark grey) in the fourth layer. x = 448 (completion of a third layer was also observed
at C6OSr236). Note that these values are identical to
the ones just predicted above for the completion of the
struct the third and fourth layers around c60 in a third and fourth layer of metal atoms. We, therefore,
manner similar to the second layer with I-symmetry: feel confident that the alkaline earth metals studied
place one atom above each of the icosahedral vertices; do, in fact, form the distinct layers around a central
for each additional layer, increase the length of the c60 molecule with the structures depicted in Fig. 5.
edges of the triangles between the vertices by one atom It should be pointed out again that these layers
with respect to the underlying layer; rotate the trian- would, of course, contain identical numbers of atoms
gles so that each edge points toward a different icosa- if the triangular faces had not been rotated and, thus,
hedral vertex. For the second layer, this angle of the Ih-symmetry had been preserved[7]. The reason
rotation is 19". For the third and fourth layer it is ap- for preferring the arrangement with I-symmetry
proximately 14" and 1 lo, respectively. These angles (which can still be called icosahedral) is that it leads
are measured relative to the position with full Ih- to higher coordination of the atoms at the borders be-
symmetry. The atoms stacked as triangular faces tween the triangular faces.
above the hexagonal rings of c60 resemble a tetra-
hedron with one tip pointing towards the center of the
cluster, having a slight twist due to the difference in
orientation of a few degrees between consecutive lay-
ers. The resulting structures of the first four layers are
depicted in Fig. 5. For clarity, one of the triangular
faces has been shaded. The atoms at the icosahedral
vertices are drawn black. The number of atoms re-
quired to complete the third and fourth layer in this
manner are 236 and 448.
At the bottom of Fig. 5, the fourth shell is shown
from two directions. Note the spiral of atoms that are
emphasized by a dark grey. This spiral can be wound
around any of the five-fold axes from tip to tip. Sim-
ilar spirals exist in the other layers, too. Each layer can 0 10000 20000
be envisioned to consist of five such spirals of atoms. mass [amu]
For each layer, there is also the stereoisomer with the
opposite sense of chirality. Fig. 6. Mass spectrum of photoionized C&a, clusters with
high metal content: additional edges, interpreted as comple-
To express the number of atoms needed to complete tion of a third and fourth layer, are observed at x = 236 and
such layers mathematically, let us introduce a layer in- x = 448.