Page 152 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 152
Polycondensation Polymers 115
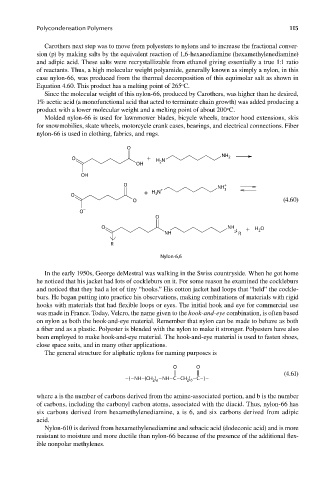
Carothers next step was to move from polyesters to nylons and to increase the fractional conver-
sion (p) by making salts by the equivalent reaction of 1,6-hexanediamine (hexamethylenediamine)
and adipic acid. These salts were recrystallizable from ethanol giving essentially a true 1:1 ratio
of reactants. Thus, a high molecular weight polyamide, generally known as simply a nylon, in this
case nylon-66, was produced from the thermal decomposition of this equimolar salt as shown in
o
Equation 4.60. This product has a melting point of 265 C.
Since the molecular weight of this nylon-66, produced by Carothers, was higher than he desired,
1% acetic acid (a monofunctional acid that acted to terminate chain growth) was added producing a
o
product with a lower molecular weight and a melting point of about 200 C.
Molded nylon-66 is used for lawnmower blades, bicycle wheels, tractor hood extensions, skis
for snowmobilies, skate wheels, motorcycle crank cases, bearings, and electrical connections. Fiber
nylon-66 is used in clothing, fabrics, and rugs.
O
O + H N NH 2
OH 2
OH
O NH +
+ H N + 3
O 3
O − (4.60)
−
O
O
O NH + H O
2
NH R
R
Nylon-6,6
In the early 1950s, George deMestral was walking in the Swiss countryside. When he got home
he noticed that his jacket had lots of cockleburs on it. For some reason he examined the cockleburs
and noticed that they had a lot of tiny “hooks.” His cotton jacket had loops that “held” the cockle-
burs. He began putting into practice his observations, making combinations of materials with rigid
hooks with materials that had flexible loops or eyes. The initial hook and eye for commercial use
was made in France. Today, Velcro, the name given to the hook-and-eye combination, is often based
on nylon as both the hook-and-eye material. Remember that nylon can be made to behave as both
a fiber and as a plastic. Polyester is blended with the nylon to make it stronger. Polyesters have also
been employed to make hook-and-eye material. The hook-and-eye material is used to fasten shoes,
close space suits, and in many other applications.
The general structure for aliphatic nylons for naming purposes is
O O
|| || (4.61)
2 a
2 b
− ( − NH −(CH ) − NH − C − CH ) − C − ) −
where a is the number of carbons derived from the amine-associated portion, and b is the number
of carbons, including the carbonyl carbon atoms, associated with the diacid. Thus, nylon-66 has
six carbons derived from hexamethylenediamine, a is 6, and six carbons derived from adipic
acid.
Nylon-610 is derived from hexamethylenediamine and sebacic acid (dodeconic acid) and is more
resistant to moisture and more ductile than nylon-66 because of the presence of the additional fl ex-
ible nonpolar methylenes.
9/14/2010 3:38:22 PM
K10478.indb 115 9/14/2010 3:38:22 PM
K10478.indb 115