Page 153 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 153
116 Carraher’s Polymer Chemistry
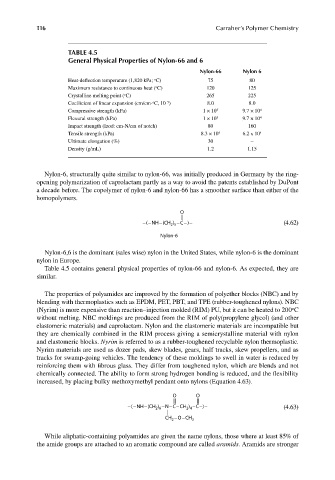
TABLE 4.5
General Physical Properties of Nylon-66 and 6
Nylon-66 Nylon 6
Heat-deflection temperature (1,820 kPa; C) 75 80
o
o
Maximum resistance to continuous heat ( C) 120 125
o
Crystalline melting point ( C) 265 225
Coefficient of linear expansion (cm/cm- C, 10 ) 8.0 8.0
o
–5
Compressive strength (kPa) 1 × 10 5 9.7 × 10 4
Flexural strength (kPa) 1 × 10 5 9.7 × 10 4
Impact strength (Izod: cm-N/cm of notch) 80 160
Tensile strength (kPa) 8.3 × 10 4 6.2 x 10 4
Ultimate elongation (%) 30 –
Density (g/mL) 1.2 1.15
Nylon-6, structurally quite similar to nylon-66, was initially produced in Germany by the ring-
opening polymerization of caprolactam partly as a way to avoid the patents established by DuPont
a decade before. The copolymer of nylon-6 and nylon-66 has a smoother surface than either of the
homopolymers.
O
||
− ( − NH − (CH ) − C − ) − (4.62)
2 5
Nylon-6
Nylon-6,6 is the dominant (sales wise) nylon in the United States, while nylon-6 is the dominant
nylon in Europe.
Table 4.5 contains general physical properties of nylon-66 and nylon-6. As expected, they are
similar.
The properties of polyamides are improved by the formation of polyether blocks (NBC) and by
blending with thermoplastics such as EPDM, PET, PBT, and TPE (rubber-toughened nylons). NBC
o
(Nyrim) is more expensive than reaction–injection molded (RIM) PU, but it can be heated to 200 C
without melting. NBC moldings are produced from the RIM of poly(propylene glycol) (and other
elastomeric materials) and caprolactam. Nylon and the elastomeric materials are incompatible but
they are chemically combined in the RIM process giving a semicrystalline material with nylon
and elastomeric blocks. Nyrim is referred to as a rubber-toughened recyclable nylon thermoplastic.
Nyrim materials are used as dozer pads, skew blades, gears, half tracks, skew propellers, and as
tracks for swamp-going vehicles. The tendency of these moldings to swell in water is reduced by
reinforcing them with fibrous glass. They differ from toughened nylon, which are blends and not
chemically connected. The ability to form strong hydrogen bonding is reduced, and the fl exibility
increased, by placing bulky methoxymethyl pendant onto nylons (Equation 4.63).
O O
|| ||
− ( − NH − (CH ) − N − C − CH ) − C −) − (4.63)
2 4
2 6
|
CH − O − CH 3
2
While aliphatic-containing polyamides are given the name nylons, those where at least 85% of
the amide groups are attached to an aromatic compound are called aramids. Aramids are stronger
K10478.indb 116
9/14/2010 3:38:23 PM
K10478.indb 116 9/14/2010 3:38:23 PM