Page 239 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 239
202 Carraher’s Polymer Chemistry
H O H 2 O H O H O H O H O
2
2
2
2
H O H O 2
2
2
H 2 O
H O H 2 O
2
H 2 O
H O
O 2 H O
H 2
O 2
H 2
H O M M M M H O
2
M
2
2
H 2 O M M H O
M M M M
M M H O
2
H 2 O
O
H 2 O
H 2
H O H 2 O
2
2
H O H O
2
H 2 O
H 2 O H 2 O H 2 O
O
H 2 H 2 O
H O MM O
2
O H 2
H 2 H O M M M
2
M M H O H O
2
2
H 2 O H O
2
H O H O H O
2
2
2
H O H O H O H O
2
2
2
H 2 O 2
H O O
H O 2 H 2 O H O H 2
2
2
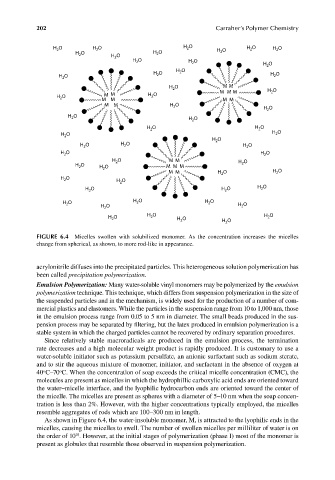
FIGURE 6.4 Micelles swollen with solubilized monomer. As the concentration increases the micelles
change from spherical, as shown, to more rod-like in appearance.
acrylonitrile diffuses into the precipitated particles. This heterogeneous solution polymerization has
been called precipitation polymerization.
Emulsion Polymerization: Many water-soluble vinyl monomers may be polymerized by the emulsion
polymerization technique. This technique, which differs from suspension polymerization in the size of
the suspended particles and in the mechanism, is widely used for the production of a number of com-
mercial plastics and elastomers. While the particles in the suspension range from 10 to 1,000 nm, those
in the emulsion process range from 0.05 to 5 nm in diameter. The small beads produced in the sus-
pension process may be separated by filtering, but the latex produced in emulsion polymerization is a
stable system in which the charged particles cannot be recovered by ordinary separation procedures.
Since relatively stable macroradicals are produced in the emulsion process, the termination
rate decreases and a high molecular weight product is rapidly produced. It is customary to use a
water-soluble initiator such as potassium persulfate, an anionic surfactant such as sodium sterate,
and to stir the aqueous mixture of monomer, initiator, and surfactant in the absence of oxygen at
40 C–70 C. When the concentration of soap exceeds the critical micelle concentration (CMC), the
o
o
molecules are present as micelles in which the hydrophillic carboxylic acid ends are oriented toward
the water–micelle interface, and the lyophilic hydrocarbon ends are oriented toward the center of
the micelle. The micelles are present as spheres with a diameter of 5–10 nm when the soap concen-
tration is less than 2%. However, with the higher concentrations typically employed, the micelles
resemble aggregates of rods which are 100–300 nm in length.
As shown in Figure 6.4, the water-insoluble monomer, M, is attracted to the lyophilic ends in the
micelles, causing the micelles to swell. The number of swollen micelles per milliliter of water is on
the order of 10 . However, at the initial stages of polymerization (phase I) most of the monomer is
18
present as globules that resemble those observed in suspension polymerization.
9/14/2010 3:39:36 PM
K10478.indb 202 9/14/2010 3:39:36 PM
K10478.indb 202