Page 240 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 240
Free Radical Chain Polymerization 203
Since the initiation of polymerization takes place in the aqueous phase, essentially no polymeri-
zation occurs in the globules. Thus, they serve primarily as a reservoir of monomer supplied to the
micelles to replace monomer converted to polymer. The number of droplets per milliliter of water is
11
on the order of 10 . Hence, since there are 10 million times as many micelles as droplets, the chance
of initiation of monomer in a droplet is very small and the chance that more than one growing chain
occurs within the same droplet is very very small.
The persulfate ion undergoes homolytic cleavage, producing two sulfate ion radicals.
−
S O → 2SO •−
2
2 8 4 (6.45)
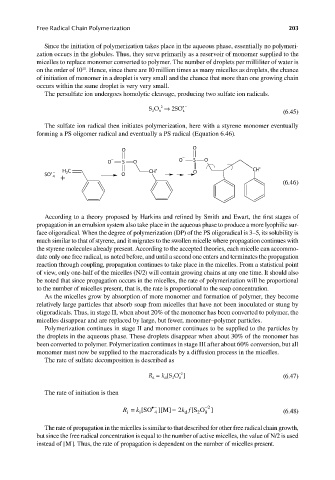
The sulfate ion radical then initiates polymerization, here with a styrene monomer eventually
forming a PS oligomer radical and eventually a PS radical (Equation 6.46).
O O
− −
O S O O S O
• − H C CH • O CH •
2
SO O
4
+
(6.46)
According to a theory proposed by Harkins and refined by Smith and Ewart, the first stages of
propagation in an emulsion system also take place in the aqueous phase to produce a more lyophilic sur-
face oligoradical. When the degree of polymerization (DP) of the PS oligoradical is 3–5, its solubility is
much similar to that of styrene, and it migrates to the swollen micelle where propagation continues with
the styrene molecules already present. According to the accepted theories, each micelle can accommo-
date only one free radical, as noted before, and until a second one enters and terminates the propagation
reaction through coupling, propagation continues to take place in the micelles. From a statistical point
of view, only one-half of the micelles (N/2) will contain growing chains at any one time. It should also
be noted that since propagation occurs in the micelles, the rate of polymerization will be proportional
to the number of micelles present, that is, the rate is proportional to the soap concentration.
As the micelles grow by absorption of more monomer and formation of polymer, they become
relatively large particles that absorb soap from micelles that have not been inoculated or stung by
oligoradicals. Thus, in stage II, when about 20% of the monomer has been converted to polymer, the
micelles disappear and are replaced by large, but fewer, monomer–polymer particles.
Polymerization continues in stage II and monomer continues to be supplied to the particles by
the droplets in the aqueous phase. These droplets disappear when about 30% of the monomer has
been converted to polymer. Polymerization continues in stage III after about 60% conversion, but all
monomer must now be supplied to the macroradicals by a diffusion process in the micelles.
The rate of sulfate decomposition is described as
−
R = k [S O ] (6.47)
2
d d 2 8
The rate of initiation is then
•− −2
R i = [SO ][M] = 2k f [S O ] (6.48)
k
2
8
d
i
4
The rate of propagation in the micelles is similar to that described for other free radical chain growth,
but since the free radical concentration is equal to the number of active micelles, the value of N/2 is used
.
instead of [M]. Thus, the rate of propagation is dependent on the number of micelles present.
9/14/2010 3:39:37 PM
K10478.indb 203 9/14/2010 3:39:37 PM
K10478.indb 203