Page 235 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 235
198 Carraher’s Polymer Chemistry
•
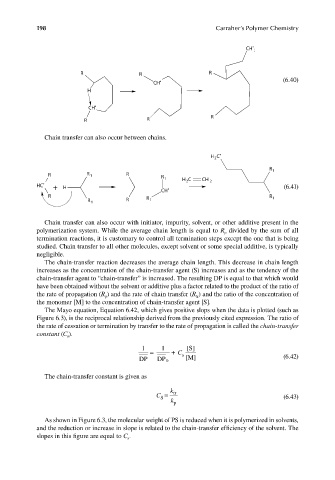
CH 2
R R R
CH • (6.40)
H
CH •
R
R R
Chain transfer can also occur between chains.
H C •
2
R 1
R R 1 R R 1 H C CH
HC • + H 2 2 (6.41)
CH •
R R R 1
R 1 R 1
Chain transfer can also occur with initiator, impurity, solvent, or other additive present in the
polymerization system. While the average chain length is equal to R divided by the sum of all
p
termination reactions, it is customary to control all termination steps except the one that is being
studied. Chain transfer to all other molecules, except solvent or some special additive, is typically
negligible.
The chain-transfer reaction decreases the average chain length. This decrease in chain length
increases as the concentration of the chain-transfer agent (S) increases and as the tendency of the
chain-transfer agent to “chain-transfer” is increased. The resulting DP is equal to that which would
have been obtained without the solvent or additive plus a factor related to the product of the ratio of
the rate of propagation (R ) and the rate of chain transfer (R ) and the ratio of the concentration of
p
tr
the monomer [M] to the concentration of chain-transfer agent [S].
The Mayo equation, Equation 6.42, which gives positive slops when the data is plotted (such as
Figure 6.3), is the reciprocal relationship derived from the previously cited expression. The ratio of
the rate of cessation or termination by transfer to the rate of propagation is called the chain-transfer
constant (C ).
s
1 1 [S]
= + C s
DP DP o [M] (6.42)
The chain-transfer constant is given as
k tr
C S = (6.43)
k p
As shown in Figure 6.3, the molecular weight of PS is reduced when it is polymerized in solvents,
and the reduction or increase in slope is related to the chain-transfer efficiency of the solvent. The
slopes in this figure are equal to C .
s
9/14/2010 3:39:34 PM
K10478.indb 198 9/14/2010 3:39:34 PM
K10478.indb 198