Page 231 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 231
194 Carraher’s Polymer Chemistry
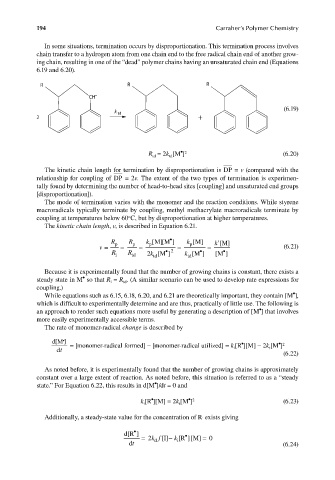
In some situations, termination occurs by disproportionation. This termination process involves
chain transfer to a hydrogen atom from one chain end to the free radical chain end of another grow-
ing chain, resulting in one of the “dead” polymer chains having an unsaturated chain end (Equations
6.19 and 6.20).
R R R
CH •
(6.19)
k td
2 +
• 2
R = 2k [M ] (6.20)
td
td
The kinetic chain length for termination by disproportionation is DP = v (compared with the
relationship for coupling of DP = 2v. The extent of the two types of termination is experimen-
tally found by determining the number of head-to-head sites [coupling] and unsaturated end groups
[disproportionation]).
The mode of termination varies with the monomer and the reaction conditions. While styrene
macroradicals typically terminate by coupling, methyl methacrylate macroradicals terminate by
o
coupling at temperatures below 60 C, but by disproportionation at higher temperatures.
The kinetic chain length, v, is described in Equation 6.21.
•
R p R p k p [M][M ] k p [M] k [M]
v = = = = • = • (6.21)
• 2
R R 2k [M] k [M] [M]
i td td td
Because it is experimentally found that the number of growing chains is constant, there exists a
•
steady state in M so that R = R . (A similar scenario can be used to develop rate expressions for
i td
coupling.)
•
While equations such as 6.15, 6.18, 6.20, and 6.21 are theoretically important, they contain [M ],
which is difficult to experimentally determine and are thus, practically of little use. The following is
•
an approach to render such equations more useful by generating a description of [M ] that involves
more easily experimentally accessible terms.
The rate of monomer-radical change is described by
•
d[M ]
•
= [monomer-radical formed] − [monomer-radical utilized] = k [R ][M] − 2k [M ]
• 2
i
t
dt
(6.22)
As noted before, it is experimentally found that the number of growing chains is approximately
constant over a large extent of reaction. As noted before, this situation is referred to as a “steady
•
state.” For Equation 6.22, this results in d[M ]/dt = 0 and
•
• 2
k [R ][M] = 2k [M ] (6.23)
i
t
.
Additionally, a steady-state value for the concentration of R exists giving
•
d[R ] •
= 2kf [I] − [R ][M]k i = 0
d
dt (6.24)
K10478.indb 194 9/14/2010 3:39:28 PM
9/14/2010 3:39:28 PM
K10478.indb 194