Page 228 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 228
Free Radical Chain Polymerization 191
6.2 MECHANISM FOR FREE RADICAL CHAIN POLYMERIZATION
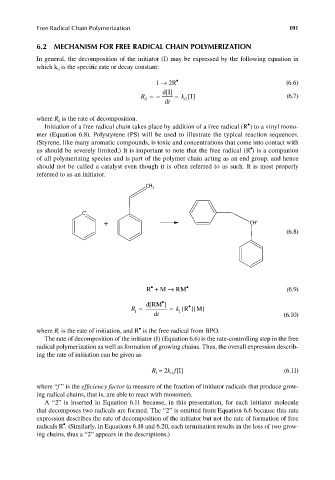
In general, the decomposition of the initiator (I) may be expressed by the following equation in
which k is the specifi c rate or decay constant:
d
I → 2R • (6.6)
d[I]
R d = – = k d [I] (6.7)
dt
where R is the rate of decomposition.
d
•
Initiation of a free radical chain takes place by addition of a free radical (R ) to a vinyl mono-
mer (Equation 6.8). Polystyrene (PS) will be used to illustrate the typical reaction sequences.
(Styrene, like many aromatic compounds, is toxic and concentrations that come into contact with
•
us should be severely limited.) It is important to note that the free radical (R ) is a companion
of all polymerizing species and is part of the polymer chain acting as an end group, and hence
should not be called a catalyst even though it is often referred to as such. It is most properly
referred to as an initiator.
CH 2
C •
+ CH •
(6.8)
•
R + M → RM • (6.9)
•
d[RM ]
•
R i = = k i [R ][M]
dt (6.10)
•
where R is the rate of initiation, and R is the free radical from BPO.
i
The rate of decomposition of the initiator (I) (Equation 6.6) is the rate-controlling step in the free
radical polymerization as well as formation of growing chains. Thus, the overall expression describ-
ing the rate of initiation can be given as
R = 2k f[I] (6.11)
i d
where “f ” is the effi ciency factor (a measure of the fraction of initiator radicals that produce grow-
ing radical chains, that is, are able to react with monomer).
A “2” is inserted in Equation 6.11 because, in this presentation, for each initiator molecule
that decomposes two radicals are formed. The “2” is omitted from Equation 6.6 because this rate
expression describes the rate of decomposition of the initiator but not the rate of formation of free
•
radicals R . (Similarly, in Equations 6.18 and 6.20, each termination results in the loss of two grow-
ing chains, thus a “2” appears in the descriptions.)
9/14/2010 3:39:22 PM
K10478.indb 191 9/14/2010 3:39:22 PM
K10478.indb 191