Page 226 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 226
Free Radical Chain Polymerization 189
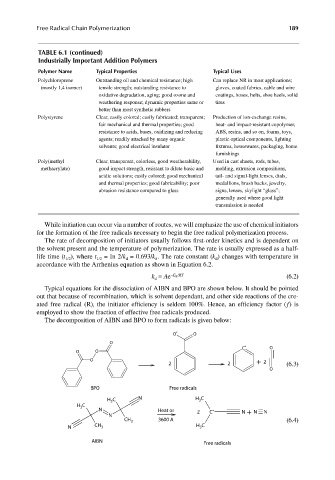
TABLE 6.1 (continued)
Industrially Important Addition Polymers
Polymer Name Typical Properties Typical Uses
Polychloroprene Outstanding oil and chemical resistance; high Can replace NR in most applications;
(mostly 1,4 isomer) tensile strength; outstanding resistance to gloves, coated fabrics, cable and wire
oxidative degradation, aging; good ozone and coatings, hoses, belts, shoe heels, solid
weathering response; dynamic properties same or tires
better than most synthetic rubbers
Polystyrene Clear, easily colored; easily fabricated; transparent; Production of ion-exchange resins,
fair mechanical and thermal properties; good heat- and impact-resistant copolymer,
resistance to acids, bases, oxidizing and reducing ABS, resins, and so on, foams, toys,
agents; readily attacked by many organic plastic optical components, lighting
solvents; good electrical insulator fixtures, housewares, packaging, home
furnishings
Poly(methyl Clear, transparent, colorless, good weatherability, Used in cast sheets, rods, tubes,
methacrylate) good impact strength, resistant to dilute basic and molding, extrusion compositions,
acidic solutions; easily colored; good mechanical tail- and signal-light lenses, dials,
and thermal properties; good fabricability; poor medallions, brush backs, jewelry,
abrasion resistance compared to glass signs, lenses, skylight “glass”;
generally used where good light
transmission is needed
While initiation can occur via a number of routes, we will emphasize the use of chemical initiators
for the formation of the free radicals necessary to begin the free radical polymerization process.
The rate of decomposition of initiators usually follows first-order kinetics and is dependent on
the solvent present and the temperature of polymerization. The rate is usually expressed as a half-
life time (t ), where t = In 2/k = 0.693/k . The rate constant (k ) changes with temperature in
1/2 1/2 d d d
accordance with the Arrhenius equation as shown in Equation 6.2.
k = Ae −E a /RT (6.2)
d
Typical equations for the dissociation of AIBN and BPO are shown below. It should be pointed
out that because of recombination, which is solvent dependant, and other side reactions of the cre-
ated free radical (R), the initiator efficiency is seldom 100%. Hence, an efficiency factor (f) is
employed to show the fraction of effective free radicals produced.
The decomposition of AIBN and BPO to form radicals is given below:
•
O O
O
•
C O
O O
O
2 2 + 2 (6.3)
O
BPO Free radicals
H C N H C
3
3
H C
3
N Heat or 2 C • N + N N
N
CH 3 3600 A (6.4)
N CH 3 H C
3
AIBN
Free radicals
9/14/2010 3:39:21 PM
K10478.indb 189
K10478.indb 189 9/14/2010 3:39:21 PM