Page 227 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 227
190 Carraher’s Polymer Chemistry
•
C
O
A
B
O
•
CH
1% 80%
•
• O CH 2
O O CH 2
O
6%
+ C
13%
O
O D
CH •
CH 2
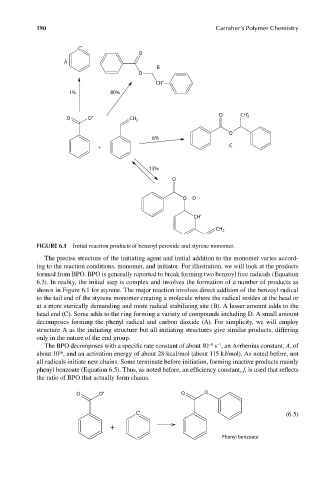
FIGURE 6.1 Initial reaction products of benzoyl peroxide and styrene monomer.
The precise structure of the initiating agent and initial addition to the monomer varies accord-
ing to the reaction conditions, monomer, and initiator. For illustration, we will look at the products
formed from BPO. BPO is generally reported to break forming two benzoyl free radicals (Equation
6.3). In reality, the initial step is complex and involves the formation of a number of products as
shown in Figure 6.1 for styrene. The major reaction involves direct addition of the benzoyl radical
to the tail end of the styrene monomer creating a molecule where the radical resides at the head or
at a more sterically demanding and more radical stabilizing site (B). A lesser amount adds to the
head end (C). Some adds to the ring forming a variety of compounds including D. A small amount
decomposes forming the phenyl radical and carbon dioxide (A). For simplicity, we will employ
structure A as the initiating structure but all initiating structures give similar products, differing
only in the nature of the end group.
–8
–1
The BPO decomposes with a specific rate constant of about 10 s , an Arrhenius constant, A, of
16
about 10 , and an activation energy of about 28 kcal/mol (about 115 kJ/mol). As noted before, not
all radicals initiate new chains. Some terminate before initiation, forming inactive products mainly
phenyl benzoate (Equation 6.5). Thus, as noted before, an effi ciency constant, f, is used that refl ects
the ratio of BPO that actually form chains.
O O • O O
C • (6.5)
+
Phenyl benzoate
9/14/2010 3:39:22 PM
K10478.indb 190 9/14/2010 3:39:22 PM
K10478.indb 190