Page 229 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 229
192 Carraher’s Polymer Chemistry
Propagation is a bimolecular reaction (Equations 6.12 and 6.13), occurring through addition of
•
a new free radical (RM ) to another molecule of monomer (M). This step is repeated many times,
resulting in the formation of the polymer chain. It is experimentally found that there may be slight
changes in the propagation rate constant (k ) in the first few steps, but the rate constant is generally
p
•
•
•
considered to be independent of chain length. Hence, the symbols M , RM , and RM M may be
n
considered equivalent in rate equations for free radical polymerization.
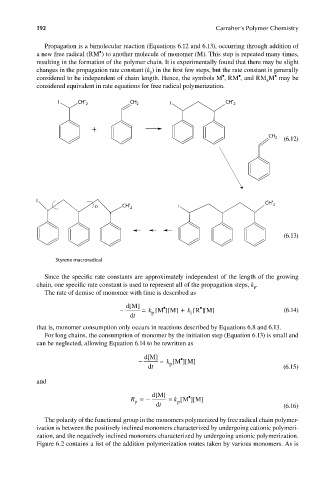
I CH • 2 CH 2 I CH • 2
+
CH 2 (6.12)
I • CH •
n CH 2 I 2
(6.13)
Styrene macroradical
Since the specific rate constants are approximately independent of the length of the growing
chain, one specific rate constant is used to represent all of the propagation steps, k .
p
The rate of demise of monomer with time is described as
d[M] • •
– = k p [M ][M] + k i [R ][M] (6.14)
dt
that is, monomer consumption only occurs in reactions described by Equations 6.8 and 6.13.
For long chains, the consumption of monomer by the initiation step (Equation 6.13) is small and
can be neglected, allowing Equation 6.14 to be rewritten as
d[M] •
− = k p [M ][M]
dt (6.15)
and
d[M] •
k
R p = − = [M ][M]
p
dt (6.16)
The polarity of the functional group in the monomers polymerized by free radical chain polymer-
ization is between the positively inclined monomers characterized by undergoing cationic polymeri-
zation, and the negatively inclined monomers characterized by undergoing anionic polymerization.
Figure 6.2 contains a list of the addition polymerization routes taken by various monomers. As is
9/14/2010 3:39:23 PM
K10478.indb 192 9/14/2010 3:39:23 PM
K10478.indb 192