Page 360 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 360
Naturally Occurring Polymers—Animals 323
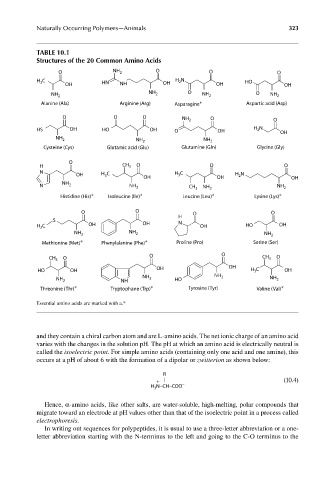
TABLE 10.1
Structures of the 20 Common Amino Acids
O NH 2 O O O
H C HN H N HO
2
3
OH NH OH OH OH
NH O
NH 2 2 NH 2 O NH 2
Alanine (Ala) Arginine (Arg) Asparagine ∗ Aspartic acid (Asp)
O O O NH 2 O O
HS OH HO OH O OH H N OH
2
NH 2 NH 2 NH 2
Cysteine (Cys) Glutamic acid (Glu) Glutamine (Gln) Glycine (Gly)
O
H CH 3 O O O
N H C H C H N
OH 3 3 2
OH OH OH
NH
N 2 NH 2 CH 3 NH 2 NH 2
∗ ∗ ∗ ∗
Histidine (His) Isoleucine (Ile) Leucine (Leu) Lysine (Lys)
O O O O
H
S N
H C OH OH OH HO OH
3
NH 2 NH 2 NH 2
∗ ∗
Methionine (Met) Phenylalanine (Phe) Proline (Pro) Serine (Ser)
CH 3 O O O CH 3 O
HO OH OH OH H 3 C OH
NH NH 2
NH 2 NH 2 HO NH 2
∗ ∗ ∗
Threonine (Thr) Tryptophane (Trp) Tyrosine (Tyr) Valine (Val)
Essential amino acids are marked with a.*
and they contain a chiral carbon atom and are L-amino acids. The net ionic charge of an amino acid
varies with the changes in the solution pH. The pH at which an amino acid is electrically neutral is
called the isoelectric point. For simple amino acids (containing only one acid and one amine), this
occurs at a pH of about 6 with the formation of a dipolar or zwitterion as shown below:
R
+ (10.4)
H N−CH−COO −
3
Hence, α-amino acids, like other salts, are water-soluble, high-melting, polar compounds that
migrate toward an electrode at pH values other than that of the isoelectric point in a process called
electrophoresis.
In writing out sequences for polypeptides, it is usual to use a three-letter abbreviation or a one-
letter abbreviation starting with the N-terminus to the left and going to the C-O terminus to the
9/14/2010 3:41:07 PM
K10478.indb 323 9/14/2010 3:41:07 PM
K10478.indb 323