Page 365 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 365
328 Carraher’s Polymer Chemistry
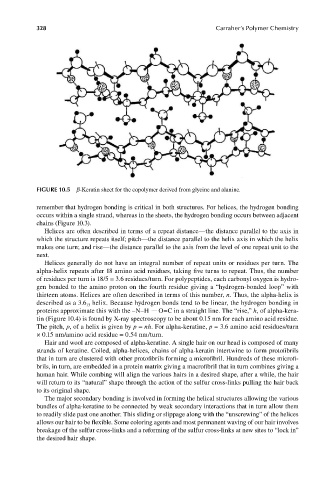
FIGURE 10.5 β-Keratin sheet for the copolymer derived from glycine and alanine.
remember that hydrogen bonding is critical in both structures. For helices, the hydrogen bonding
occurs within a single strand, whereas in the sheets, the hydrogen bonding occurs between adjacent
chains (Figure 10.3).
Helices are often described in terms of a repeat distance—the distance parallel to the axis in
which the structure repeats itself; pitch—the distance parallel to the helix axis in which the helix
makes one turn; and rise—the distance parallel to the axis from the level of one repeat unit to the
next.
Helices generally do not have an integral number of repeat units or residues per turn. The
alpha-helix repeats after 18 amino acid residues, taking five turns to repeat. Thus, the number
of residues per turn is 18/5 = 3.6 residues/turn. For polypeptides, each carbonyl oxygen is hydro-
gen bonded to the amino proton on the fourth residue giving a “hydrogen-bonded loop” with
thirteen atoms. Helices are often described in terms of this number, n. Thus, the alpha-helix is
described as a 3.6 helix. Because hydrogen bonds tend to be linear, the hydrogen bonding in
13
......
proteins approximate this with the –N–H O=C in a straight line. The “rise,” h, of alpha-kera-
tin (Figure 10.4) is found by X-ray spectroscopy to be about 0.15 nm for each amino acid residue.
The pitch, p, of a helix is given by p = nh. For alpha-keratine, p = 3.6 amino acid residues/turn
× 0.15 nm/amino acid residue = 0.54 nm/turn.
Hair and wool are composed of alpha-keratine. A single hair on our head is composed of many
strands of keratine. Coiled, alpha-helices, chains of alpha-keratin intertwine to form protofi brils
that in turn are clustered with other protofibrils forming a microfibril. Hundreds of these microfi -
brils, in turn, are embedded in a protein matrix giving a macrofibril that in turn combines giving a
human hair. While combing will align the various hairs in a desired shape, after a while, the hair
will return to its “natural” shape through the action of the sulfur cross-links pulling the hair back
to its original shape.
The major secondary bonding is involved in forming the helical structures allowing the various
bundles of alpha-keratine to be connected by weak secondary interactions that in turn allow them
to readily slide past one another. This sliding or slippage along with the “unscrewing” of the helices
allows our hair to be flexible. Some coloring agents and most permanent waving of our hair involves
breakage of the sulfur cross-links and a reforming of the sulfur cross-links at new sites to “lock in”
the desired hair shape.
9/14/2010 3:41:11 PM
K10478.indb 328 9/14/2010 3:41:11 PM
K10478.indb 328