Page 419 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 419
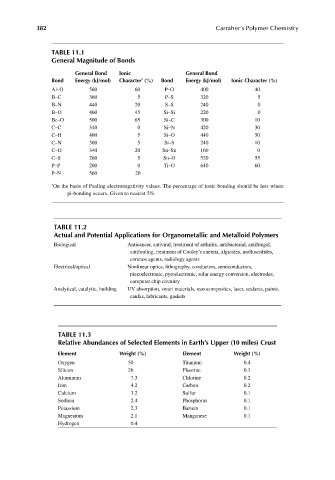
382 Carraher’s Polymer Chemistry
TABLE 11.1
General Magnitude of Bonds
General Bond Ionic General Bond
Bond Energy (kJ/mol) Character (%) Bond Energy (kJ/mol) Ionic Character (%)
*
Al–O 560 60 P–O 400 40
B–C 360 5 P–S 320 5
B–N 440 20 S–S 240 0
B–O 460 45 Si–Si 220 0
Be–O 500 65 Si–C 300 10
C–C 340 0 Si–N 420 30
C–H 400 5 Si–O 440 50
C–N 300 5 Si–S 240 10
C–O 340 20 Sn–Sn 160 0
C–S 260 5 Sn–O 520 55
P–P 200 0 Ti–O 640 60
P–N 560 20
* On the basis of Pauling electronegativity values. The percentage of ionic bonding should be less where
pi-bonding occurs. Given to nearest 5%.
TABLE 11.2
Actual and Potential Applications for Organometallic and Metalloid Polymers
Biological Anticancer, antiviral, treatment of arthritis, antibacterial, antifungal,
antifouling, treatment of Cooley’s anemia, algicides, molluscidides,
contrast agents, radiology agents
Electrical/optical Nonlinear optics, lithography, conductors, semiconductors,
piezoelectronic, pyroelectronic, solar energy conversion, electrodes,
computer chip circuitry
Analytical, catalytic, building UV absorption, smart materials, nanocomposites, laser, sealants, paints,
caulks, lubricants, gaskets
TABLE 11.3
Relative Abundances of Selected Elements in Earth’s Upper (10 miles) Crust
Element Weight (%) Element Weight (%)
Oxygen 50 Titanium 0.4
Silicon 26 Fluorine 0.3
Aluminum 7.3 Chlorine 0.2
Iron 4.2 Carbon 0.2
Calcium 3.2 Sulfur 0.1
Sodium 2.4 Phosphorus 0.1
Potassium 2.3 Barium 0.1
Magnesium 2.1 Manganese 0.1
Hydrogen 0.4
9/14/2010 3:41:28 PM
K10478.indb 382 9/14/2010 3:41:28 PM
K10478.indb 382