Page 423 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 423
386 Carraher’s Polymer Chemistry
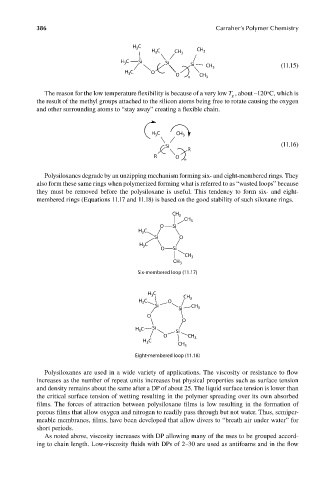
H 3 C
H C CH 3 CH 3
3
C Si
H 3 Si Si CH 3 (11.15)
H C O O
3
n CH 3
o
The reason for the low temperature flexibility is because of a very low T , about –120 C, which is
g
the result of the methyl groups attached to the silicon atoms being free to rotate causing the oxygen
and other surrounding atoms to “stay away” creating a fl exible chain.
H C CH 3
3
Si (11.16)
R
R O
n
Polysiloxanes degrade by an unzipping mechanism forming six- and eight-membered rings. They
also form these same rings when polymerized forming what is referred to as “wasted loops” because
they must be removed before the polysiloxane is useful. This tendency to form six- and eight-
membered rings (Equations 11.17 and 11.18) is based on the good stability of such siloxane rings.
CH 3
CH 3
O Si
H C
3
Si O
H 3 C
O Si
CH 3
CH 3
Six-membered loop (11.17)
H C CH
3
H C O 3
3
Si CH
Si 3
O
O
H C Si Si
3
O CH 3
H C CH 3
3
Eight-membered loop (11.18)
Polysiloxanes are used in a wide variety of applications. The viscosity or resistance to fl ow
increases as the number of repeat units increases but physical properties such as surface tension
and density remains about the same after a DP of about 25. The liquid surface tension is lower than
the critical surface tension of wetting resulting in the polymer spreading over its own absorbed
films. The forces of attraction between polysiloxane films is low resulting in the formation of
porous films that allow oxygen and nitrogen to readily pass through but not water. Thus, semiper-
meable membranes, films, have been developed that allow divers to “breath air under water” for
short periods.
As noted above, viscosity increases with DP allowing many of the uses to be grouped accord-
ing to chain length. Low-viscosity fluids with DPs of 2–30 are used as antifoams and in the fl ow
9/14/2010 3:41:37 PM
K10478.indb 386 9/14/2010 3:41:37 PM
K10478.indb 386