Page 428 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 428
Organometallic and Inorganic–Organic Polymers 391
N
H 2 NH 2
+
Ti
Cl Cl +
N O
–
O
O
+ –
N O
NH NH
R Ti R
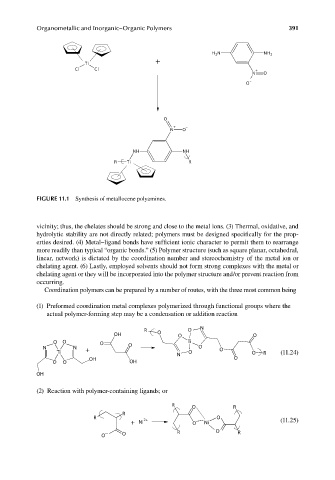
FIGURE 11.1 Synthesis of metallocene polyamines.
vicinity; thus, the chelates should be strong and close to the metal ions. (3) Thermal, oxidative, and
hydrolytic stability are not directly related; polymers must be designed specifically for the prop-
erties desired. (4) Metal–ligand bonds have sufficient ionic character to permit them to rearrange
more readily than typical “organic bonds.” (5) Polymer structure (such as square planar, octahedral,
linear, network) is dictated by the coordination number and stereochemistry of the metal ion or
chelating agent. (6) Lastly, employed solvents should not form strong complexes with the metal or
chelating agent or they will be incorporated into the polymer structure and/or prevent reaction from
occurring.
Coordination polymers can be prepared by a number of routes, with the three most common being
(1) Preformed coordination metal complexes polymerized through functional groups where the
actual polymer-forming step may be a condensation or addition reaction
R O N
OH O O O
O O O O Ti
N N + O O
Ti O O R (11.24)
N
OH O
O O OH
OH
(2) Reaction with polymer-containing ligands; or
R O R
R
R 2+ O (11.25)
+ Ni O Ni
− O R O R
O
9/14/2010 3:41:40 PM
K10478.indb 391 9/14/2010 3:41:40 PM
K10478.indb 391