Page 429 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 429
392 Carraher’s Polymer Chemistry
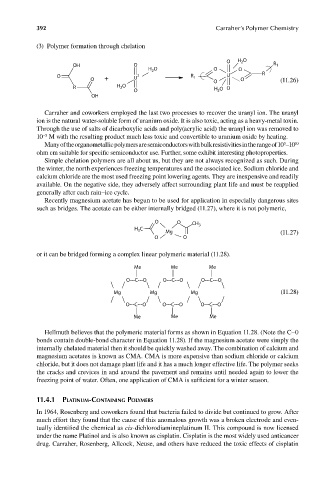
(3) Polymer formation through chelation
O H O
2
OH O R 1
H O O O
2
O 2− R 1 U R
O + U O O (11.26)
R H 2 O H O O
O 2
OH
Carraher and coworkers employed the last two processes to recover the uranyl ion. The uranyl
ion is the natural water-soluble form of uranium oxide. It is also toxic, acting as a heavy-metal toxin.
Through the use of salts of dicarboxylic acids and poly(acrylic acid) the uranyl ion was removed to
–5
10 M with the resulting product much less toxic and convertible to uranium oxide by heating.
10
Many of the organometallic polymers are semiconductors with bulk resistivities in the range of 10 –10
3
ohm cm suitable for specific semiconductor use. Further, some exhibit interesting photoproperties.
Simple chelation polymers are all about us, but they are not always recognized as such. During
the winter, the north experiences freezing temperatures and the associated ice. Sodium chloride and
calcium chloride are the most used freezing point lowering agents. They are inexpensive and readily
available. On the negative side, they adversely affect surrounding plant life and must be reapplied
generally after each rain–ice cycle.
Recently magnesium acetate has begun to be used for application in especially dangerous sites
such as bridges. The acetate can be either internally bridged (11.27), where it is not polymeric,
O O CH
C 3
H 3
Mg (11.27)
O O
or it can be bridged forming a complex linear polymeric material (11.28).
Me Me Me
O---C---O O---C---O O---C---O
Mg Mg Mg (11.28)
O---C---O O---C---O O---C---O
Me Me Me
Hellmuth believes that the polymeric material forms as shown in Equation 11.28. (Note the C–0
bonds contain double-bond character in Equation 11.28). If the magnesium acetate were simply the
internally chelated material then it should be quickly washed away. The combination of calcium and
magnesium acetates is known as CMA. CMA is more expensive than sodium chloride or calcium
chloride, but it does not damage plant life and it has a much longer effective life. The polymer seeks
the cracks and crevices in and around the pavement and remains until needed again to lower the
freezing point of water. Often, one application of CMA is sufficient for a winter season.
11.4.1 PLATINUM-CONTAINING POLYMERS
In 1964, Rosenberg and coworkers found that bacteria failed to divide but continued to grow. After
much effort they found that the cause of this anomalous growth was a broken electrode and even-
tually identified the chemical as cis-dichlorodiamineplatinum II. This compound is now licensed
under the name Platinol and is also known as cisplatin. Cisplatin is the most widely used anticancer
drug. Carraher, Rosenberg, Allcock, Neuse, and others have reduced the toxic effects of cisplatin
K10478.indb 392 9/14/2010 3:41:41 PM
9/14/2010 3:41:41 PM
K10478.indb 392