Page 431 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 431
394 Carraher’s Polymer Chemistry
Much of the interest in the polysilanes, polygermanes, and polystannanes involves their sigma
delocalization and their sigma-pi delocalization when coupled with arenes or acetylenes. This is not
unexpected since silicon exists as a covalent network similar to diamond. In exhibiting electrical
conductivity, germanium and tin show more typical “metallic” bonding. Some polystannanes have
been referred to as “molecular metals.”
Because of the interesting electronic and physical properties of polysilanes, a number of poten-
tial uses have been suggested, including precursors of beta-SiC fibers, impregnation of ceramics,
polymerization initiators, photoconductors for electrophotography, contrast enhancement layers in
photolithography, deep UV-sensitive photoresists, nonlinear optical materials, and self-developing
by excimer laser for deep UV exposure. The unusual absorption spectra of polysilanes have indi-
cated potential use in a number of conducting areas.
One area of active interest in ceramics is the formation of ceramics that may contain some fi ber
structure. Currently, ceramics, while very strong, are very brittle. Introduction of thermally stable
fiber-like materials might allow the ceramics some flexibility before cleavage. Such materials might
be considered as ceramic composites where the matrix is the ceramic portion and the fi bers are
the thermally stable fibers. Introduction of the fibers during the ceramic-forming step is a major
obstacle that must be overcome. Carbon fibers have been investigated as have been other high-
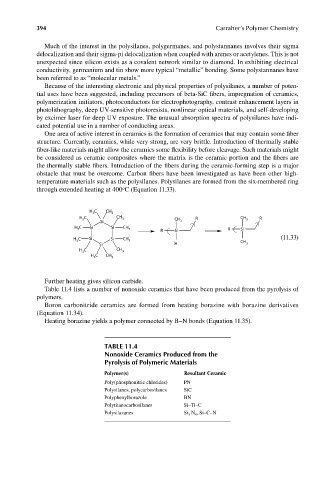
temperature materials such as the polysilanes. Polysilanes are formed from the six-membered ring
o
through extended heating at 400 C (Equation 11.33).
H C CH
3 3
H C CH 3 CH R CH 3 R
3
Si 3
H C Si Si CH 3 R Si R Si
3
C Si Si CH (11.33)
H 3 3 CH
Si H 3
C CH
H 3 3
H 3 C CH 3
Further heating gives silicon carbide.
Table 11.4 lists a number of nonoxide ceramics that have been produced from the pyrolysis of
polymers.
Boron carbonitride ceramics are formed from heating borazine with borazine derivatives
(Equation 11.34).
Heating borazine yields a polymer connected by B–N bonds (Equation 11.35).
TABLE 11.4
Nonoxide Ceramics Produced from the
Pyrolysis of Polymeric Materials
Polymer(s) Resultant Ceramic
Poly(phosphonitric chlorides) PN
Polysilanes, polycarbosilanes SiC
Polyphenylborazole BN
Polytitanocarbosilanes Si–Ti–C
Polysilazanes Si 3 N 4 , Si–C–N
9/14/2010 3:41:43 PM
K10478.indb 394 9/14/2010 3:41:43 PM
K10478.indb 394